Note
This page was generated from
scarches_scvi_tools.ipynb.
Interactive online version:
.
Some tutorial content may look better in light mode.
Reference mapping with scvi-tools#
This tutorial covers the usage of the scArches method with SCVI, SCANVI, and TOTALVI.
This particular workflow is useful in the case where a model is trained on some data (called reference here) and new samples are received (called query). The goal is to analyze these samples in the context of the reference, by mapping the query cells to the same reference latent space. This workflow may also be used in the scarches package, but here we demonstrate using only scvi-tools.
Imports and scvi-tools installation (colab)#
[ ]:
!pip install --quiet scvi-colab
import sys
from scvi_colab import install
install()
IN_COLAB = "google.colab" in sys.modules
if IN_COLAB:
!pip install --quiet scrublet
[1]:
import sys
import warnings
import anndata
import matplotlib.pyplot as plt
import numpy as np
import pandas as pd
import scanpy as sc
import scrublet as scr
import scvi
|████████████████████████████████| 229 kB 4.1 MB/s
|████████████████████████████████| 51 kB 6.3 MB/s
INFO scvi-colab: Installing scvi-tools.
INFO scvi-colab: Install successful. Testing import.
Global seed set to 0
|████████████████████████████████| 646 kB 4.3 MB/s
Building wheel for annoy (setup.py) ... done
[2]:
warnings.simplefilter(action="ignore", category=FutureWarning)
sc.set_figure_params(figsize=(4, 4))
scvi.settings.seed = 94705
%config InlineBackend.print_figure_kwargs={'facecolor' : "w"}
%config InlineBackend.figure_format='retina'
Global seed set to 94705
Reference mapping with SCVI#
Here we use the pancreas dataset described in the scIB manuscript, that is also widely used to benchmark integration methods.
[3]:
url = "https://figshare.com/ndownloader/files/24539828"
adata = sc.read("pancreas.h5ad", backup_url=url)
print(adata)
AnnData object with n_obs × n_vars = 16382 × 19093
obs: 'tech', 'celltype', 'size_factors'
layers: 'counts'
[4]:
adata.obs.tech.value_counts()
[4]:
inDrop3 3605
smartseq2 2394
celseq2 2285
inDrop1 1937
inDrop2 1724
smarter 1492
inDrop4 1303
celseq 1004
fluidigmc1 638
Name: tech, dtype: int64
We consider the SS2 and CelSeq2 samples as query, and all the others as reference.
[5]:
query = np.array([s in ["smartseq2", "celseq2"] for s in adata.obs.tech])
adata_ref = adata[~query].copy()
adata_query = adata[query].copy()
We run highly variable gene selection on the reference data and use these same genes for the query data.
[6]:
sc.pp.highly_variable_genes(adata_ref, n_top_genes=2000, batch_key="tech", subset=True)
adata_query = adata_query[:, adata_ref.var_names].copy()
/usr/local/lib/python3.7/dist-packages/pandas/core/indexing.py:1732: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
self._setitem_single_block(indexer, value, name)
Train reference#
We train the reference using the standard SCVI workflow, except we add a few non-default parameters that were identified to work well with scArches.
[7]:
scvi.model.SCVI.setup_anndata(adata_ref, batch_key="tech", layer="counts")
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
[8]:
arches_params = dict(
use_layer_norm="both",
use_batch_norm="none",
encode_covariates=True,
dropout_rate=0.2,
n_layers=2,
)
vae_ref = scvi.model.SCVI(adata_ref, **arches_params)
vae_ref.train()
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
/usr/local/lib/python3.7/dist-packages/pytorch_lightning/trainer/connectors/data_connector.py:489: PossibleUserWarning: Your `val_dataloader`'s sampler has shuffling enabled, it is strongly recommended that you turn shuffling off for val/test/predict dataloaders.
category=PossibleUserWarning,
Epoch 1/400: 0%| | 0/400 [00:00<?, ?it/s]
/usr/local/lib/python3.7/dist-packages/scvi/distributions/_negative_binomial.py:438: UserWarning: The value argument must be within the support of the distribution
UserWarning,
Epoch 400/400: 100%|██████████| 400/400 [06:31<00:00, 1.02it/s, loss=768, v_num=1]
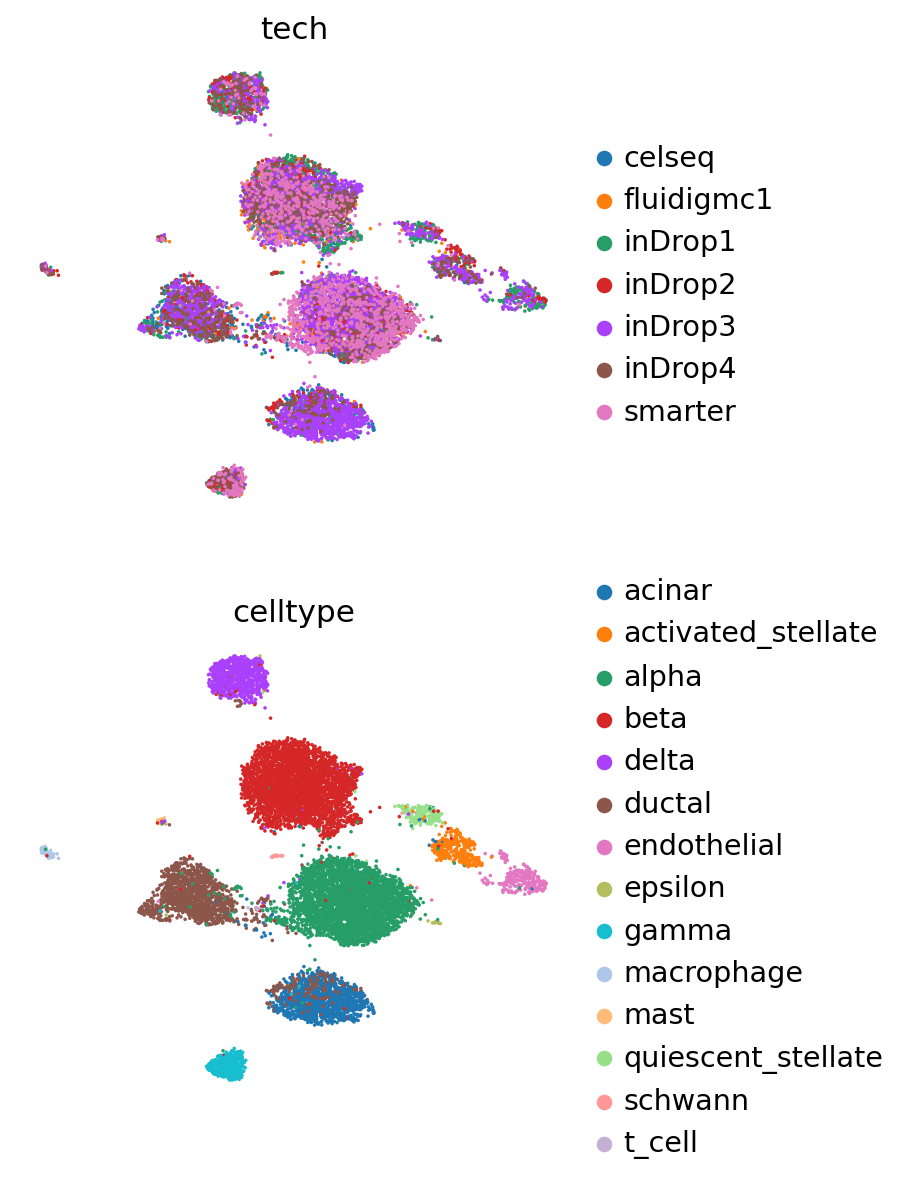
Now we obtain the latent representation, and use Scanpy to visualize with UMAP.
[9]:
adata_ref.obsm["X_scVI"] = vae_ref.get_latent_representation()
sc.pp.neighbors(adata_ref, use_rep="X_scVI")
sc.tl.leiden(adata_ref)
sc.tl.umap(adata_ref)
/usr/local/lib/python3.7/dist-packages/numba/np/ufunc/parallel.py:363: NumbaWarning: The TBB threading layer requires TBB version 2019.5 or later i.e., TBB_INTERFACE_VERSION >= 11005. Found TBB_INTERFACE_VERSION = 9107. The TBB threading layer is disabled.
warnings.warn(problem)
[10]:
sc.pl.umap(
adata_ref,
color=["tech", "celltype"],
frameon=False,
ncols=1,
)
Update with query#
We can load a new model with the query data either using
The saved reference model
The instance of the reference model
[11]:
# save the reference model
dir_path = "pancreas_model/"
vae_ref.save(dir_path, overwrite=True)
First we validate that our query data is ready to be loaded into the reference model. Here we run prepare_query_anndata, which reorders the genes and pads any missing genes with 0s. This should generally be run before reference mapping with scArches to ensure data correctness. In the case of this tutorial, nothing happens as the query data is already “correct”.
[12]:
# both are valid
scvi.model.SCVI.prepare_query_anndata(adata_query, dir_path)
scvi.model.SCVI.prepare_query_anndata(adata_query, vae_ref)
INFO File pancreas_model/model.pt already downloaded
INFO Found 100.0% reference vars in query data.
INFO Found 100.0% reference vars in query data.
Now we create the new query model instance.
[13]:
# both are valid
vae_q = scvi.model.SCVI.load_query_data(
adata_query,
dir_path,
)
vae_q = scvi.model.SCVI.load_query_data(
adata_query,
vae_ref,
)
INFO File pancreas_model/model.pt already downloaded
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
This is a typical SCVI object, and after training, can be used in any defined way.
For training the query data, we recommend using a weight_decay of 0.0. This ensures the latent representation of the reference cells will remain exactly the same if passing them through this new query model.
[14]:
vae_q.train(max_epochs=200, plan_kwargs=dict(weight_decay=0.0))
adata_query.obsm["X_scVI"] = vae_q.get_latent_representation()
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
/usr/local/lib/python3.7/dist-packages/pytorch_lightning/trainer/connectors/data_connector.py:489: PossibleUserWarning: Your `val_dataloader`'s sampler has shuffling enabled, it is strongly recommended that you turn shuffling off for val/test/predict dataloaders.
category=PossibleUserWarning,
Epoch 1/200: 0%| | 0/200 [00:00<?, ?it/s]
/usr/local/lib/python3.7/dist-packages/scvi/distributions/_negative_binomial.py:438: UserWarning: The value argument must be within the support of the distribution
UserWarning,
Epoch 200/200: 100%|██████████| 200/200 [01:15<00:00, 2.63it/s, loss=1.76e+03, v_num=1]
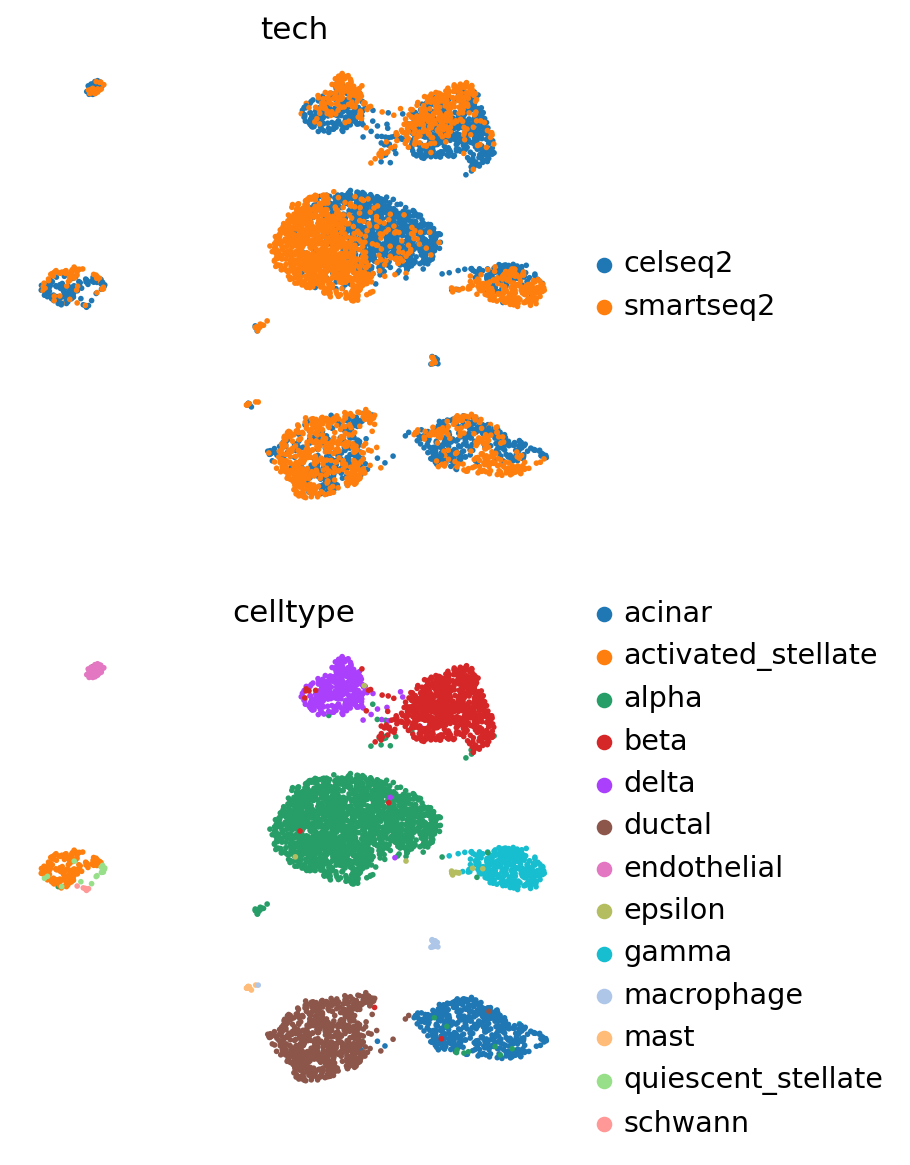
[15]:
sc.pp.neighbors(adata_query, use_rep="X_scVI")
sc.tl.leiden(adata_query)
sc.tl.umap(adata_query)
[16]:
sc.pl.umap(
adata_query,
color=["tech", "celltype"],
frameon=False,
ncols=1,
)
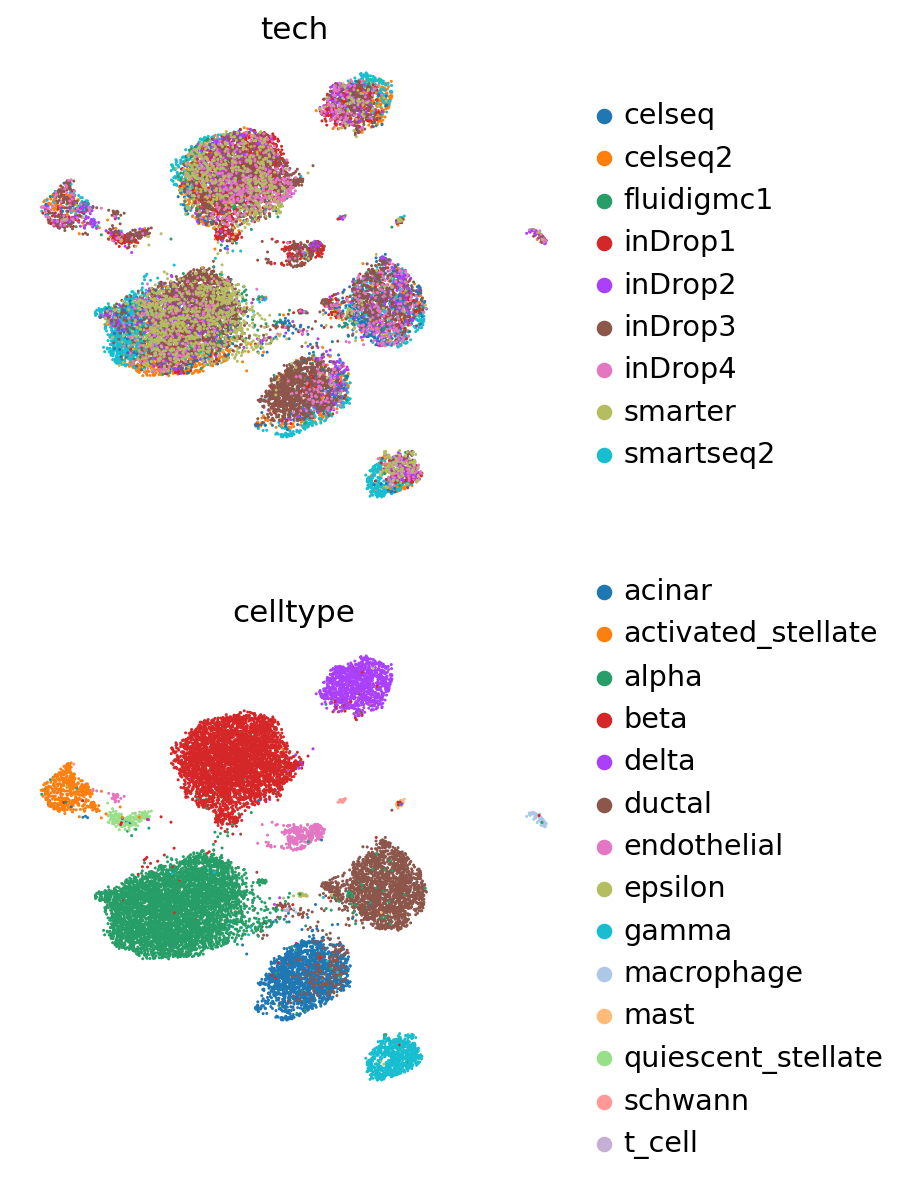
Visualize reference and query#
[17]:
adata_full = adata_query.concatenate(adata_ref)
The concatenated object has the latent representations of both reference and query, but we are also able to reobtain these values using the query model.
[18]:
adata_full.obsm["X_scVI"] = vae_q.get_latent_representation(adata_full)
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
[19]:
sc.pp.neighbors(adata_full, use_rep="X_scVI")
sc.tl.leiden(adata_full)
sc.tl.umap(adata_full)
[20]:
sc.pl.umap(
adata_full,
color=["tech", "celltype"],
frameon=False,
ncols=1,
)
Reference mapping with SCANVI#
We’ll use the same Pancreas dataset, this time we set it up such that we register that the dataset has labels.
The advantage of SCANVI is that we’ll be able to predict the cell type labels of the query dataset. In the case of SCVI, a separate classifier (e.g., nearest-neighbor, random forest, etc.) would have to be trained on the reference latent space.
Train reference#
SCANVI tends to perform better in situations where it has been initialized using a pre-trained SCVI model. In this case, we will use vae_ref that we have already trained above. In other words, a typical SCANVI workflow will be:
scvi_model = SCVI(adata_ref, **arches_params)
scvi_model.train()
scanvi_model = SCANVI.from_scvi_model(scvi_model, unlabeled_category="Unknown")
scanvi_model.train()
SCANVI.from_scvi_model will also run setup_anndata. It will use the batch_key and layer used with SCVI, but here we add the labels_key.
For this part of the tutorial, we will create a new labels key in the reference anndata object to reflect the common scenario of having no labels for the query data.
[21]:
adata_ref.obs["labels_scanvi"] = adata_ref.obs["celltype"].values
Applying this workflow in the context of this tutorial:
[22]:
# unlabeled category does not exist in adata.obs[labels_key]
# so all cells are treated as labeled
vae_ref_scan = scvi.model.SCANVI.from_scvi_model(
vae_ref,
unlabeled_category="Unknown",
labels_key="labels_scanvi",
)
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
[23]:
vae_ref_scan.train(max_epochs=20, n_samples_per_label=100)
INFO Training for 20 epochs.
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
Epoch 1/20: 0%| | 0/20 [00:00<?, ?it/s]
/usr/local/lib/python3.7/dist-packages/scvi/distributions/_negative_binomial.py:438: UserWarning: The value argument must be within the support of the distribution
UserWarning,
Epoch 20/20: 100%|██████████| 20/20 [00:40<00:00, 2.05s/it, loss=851, v_num=1]
[24]:
adata_ref.obsm["X_scANVI"] = vae_ref_scan.get_latent_representation()
sc.pp.neighbors(adata_ref, use_rep="X_scANVI")
sc.tl.leiden(adata_ref)
sc.tl.umap(adata_ref)
[25]:
sc.pl.umap(
adata_ref,
color=["tech", "celltype"],
frameon=False,
ncols=1,
)
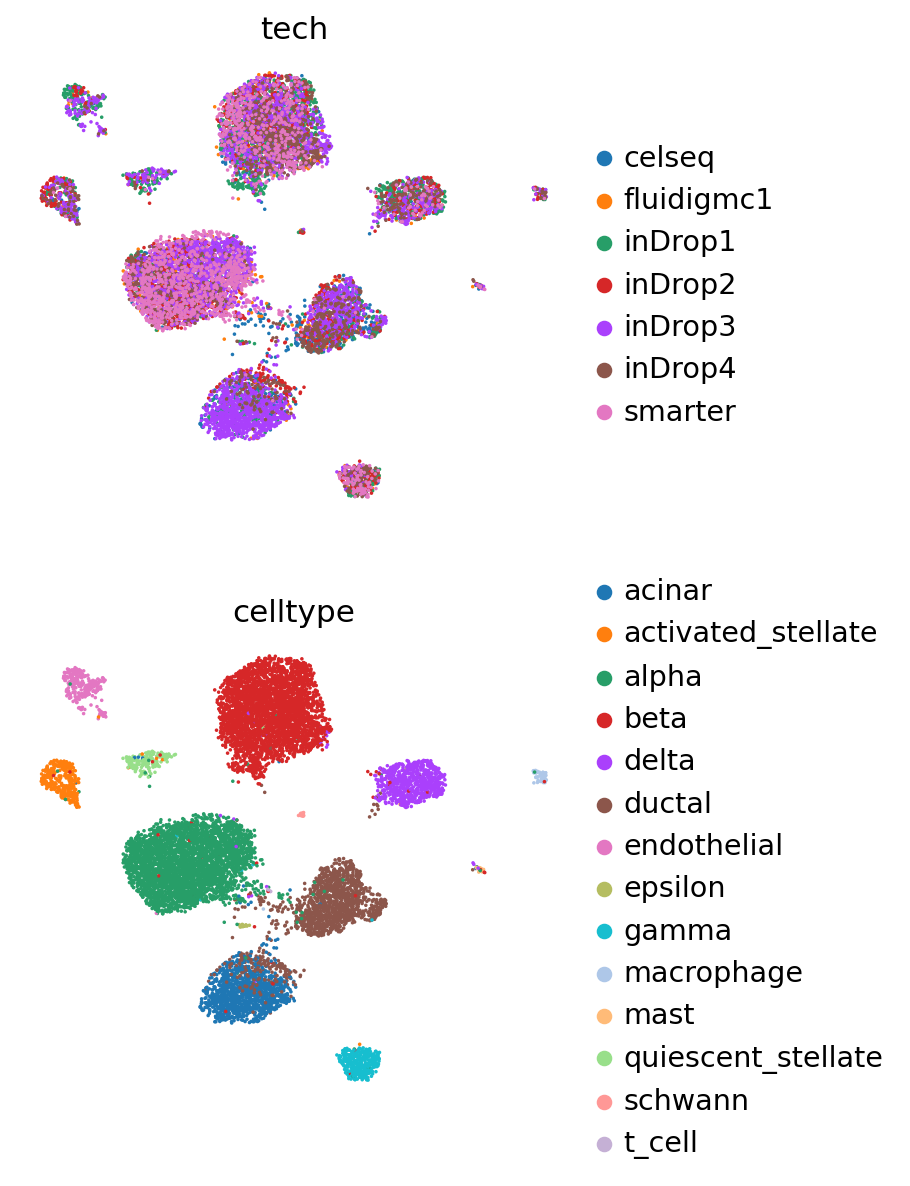
Update with query#
[26]:
dir_path_scan = "pancreas_model_scanvi/"
vae_ref_scan.save(dir_path_scan, overwrite=True)
[27]:
# again a no-op in this tutorial, but good practice to use
scvi.model.SCANVI.prepare_query_anndata(adata_query, dir_path_scan)
INFO File pancreas_model_scanvi/model.pt already downloaded
INFO Found 100.0% reference vars in query data.
Notice that adata_query.obs["labels_scanvi"] does not exist. The load_query_data method detects this and fills it in adata_query with the unlabeled category (here "Unknown").
[28]:
vae_q = scvi.model.SCANVI.load_query_data(
adata_query,
dir_path_scan,
)
INFO File pancreas_model_scanvi/model.pt already downloaded
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_scanvi.py:90: UserWarning: Missing labels key labels_scanvi. Filling in with unlabeled category Unknown.
f"Missing labels key {self._original_attr_key}. Filling in with unlabeled category {self._unlabeled_category}."
[29]:
vae_q.train(
max_epochs=100,
plan_kwargs=dict(weight_decay=0.0),
check_val_every_n_epoch=10,
)
INFO Training for 100 epochs.
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
/usr/local/lib/python3.7/dist-packages/pytorch_lightning/trainer/connectors/data_connector.py:489: PossibleUserWarning: Your `val_dataloader`'s sampler has shuffling enabled, it is strongly recommended that you turn shuffling off for val/test/predict dataloaders.
category=PossibleUserWarning,
Epoch 1/100: 0%| | 0/100 [00:00<?, ?it/s]
/usr/local/lib/python3.7/dist-packages/scvi/distributions/_negative_binomial.py:438: UserWarning: The value argument must be within the support of the distribution
UserWarning,
Epoch 100/100: 100%|██████████| 100/100 [01:02<00:00, 1.60it/s, loss=1.78e+03, v_num=1]
[30]:
adata_query.obsm["X_scANVI"] = vae_q.get_latent_representation()
adata_query.obs["predictions"] = vae_q.predict()
[31]:
df = adata_query.obs.groupby(["celltype", "predictions"]).size().unstack(fill_value=0)
norm_df = df / df.sum(axis=0)
plt.figure(figsize=(8, 8))
_ = plt.pcolor(norm_df)
_ = plt.xticks(np.arange(0.5, len(df.columns), 1), df.columns, rotation=90)
_ = plt.yticks(np.arange(0.5, len(df.index), 1), df.index)
plt.xlabel("Predicted")
plt.ylabel("Observed")
[31]:
Text(0, 0.5, 'Observed')
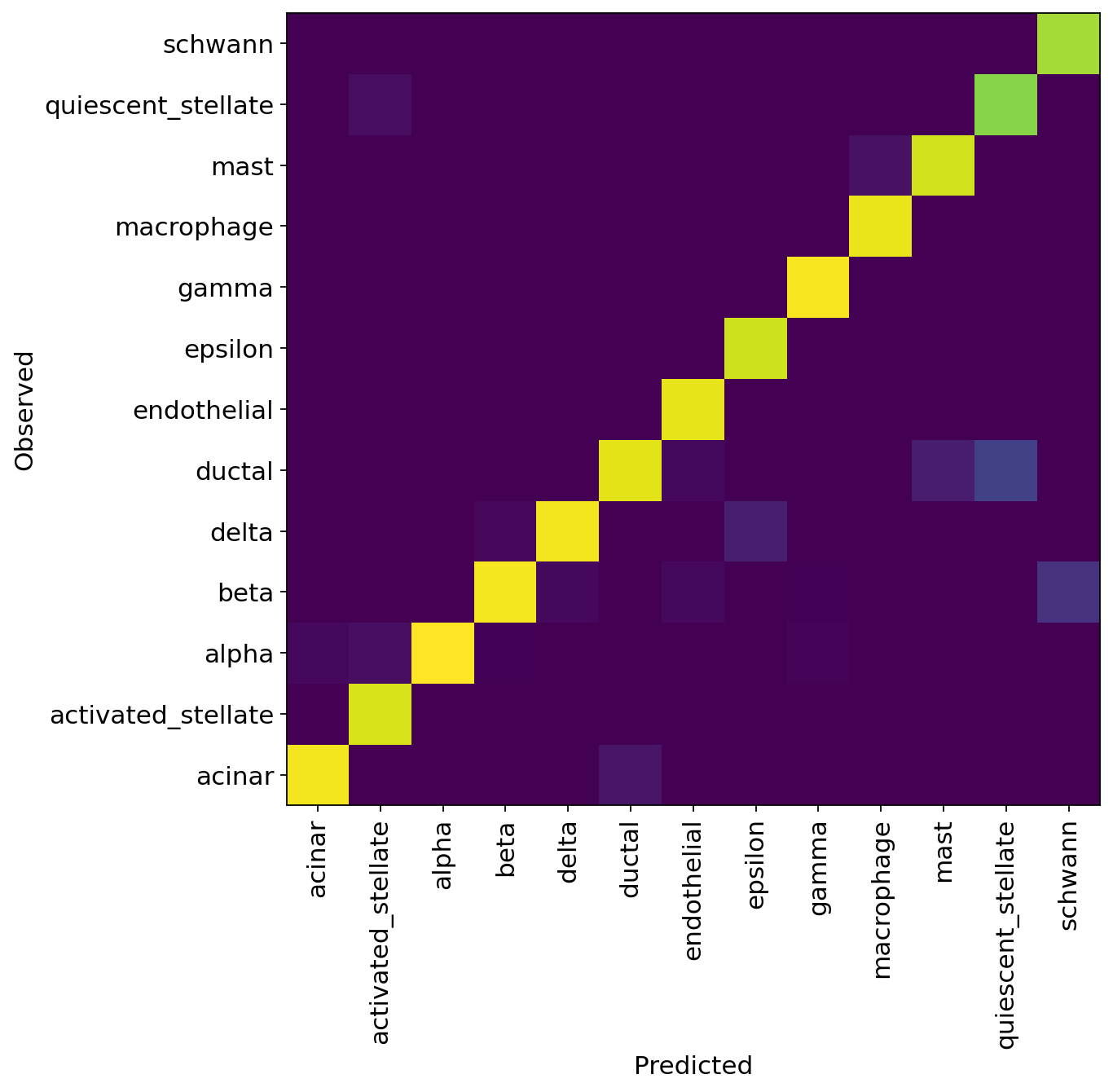
Analyze reference and query#
[32]:
adata_full = adata_query.concatenate(adata_ref)
This just makes a column in the anndata corresponding to if the data come from the reference or query sets.
[33]:
adata_full.obs.batch.cat.rename_categories(["Query", "Reference"], inplace=True)
[34]:
full_predictions = vae_q.predict(adata_full)
print(f"Acc: {np.mean(full_predictions == adata_full.obs.celltype)}")
adata_full.obs["predictions"] = full_predictions
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
/usr/local/lib/python3.7/dist-packages/scvi/data/fields/_layer_field.py:79: UserWarning: adata.layers[counts] does not contain unnormalized count data. Are you sure this is what you want?
f"{logger_data_loc} does not contain unnormalized count data. "
Acc: 0.9651446709803443
[35]:
sc.pp.neighbors(adata_full, use_rep="X_scANVI")
sc.tl.leiden(adata_full)
sc.tl.umap(adata_full)
[36]:
sc.pl.umap(
adata_full,
color=["tech", "celltype"],
frameon=False,
ncols=1,
)
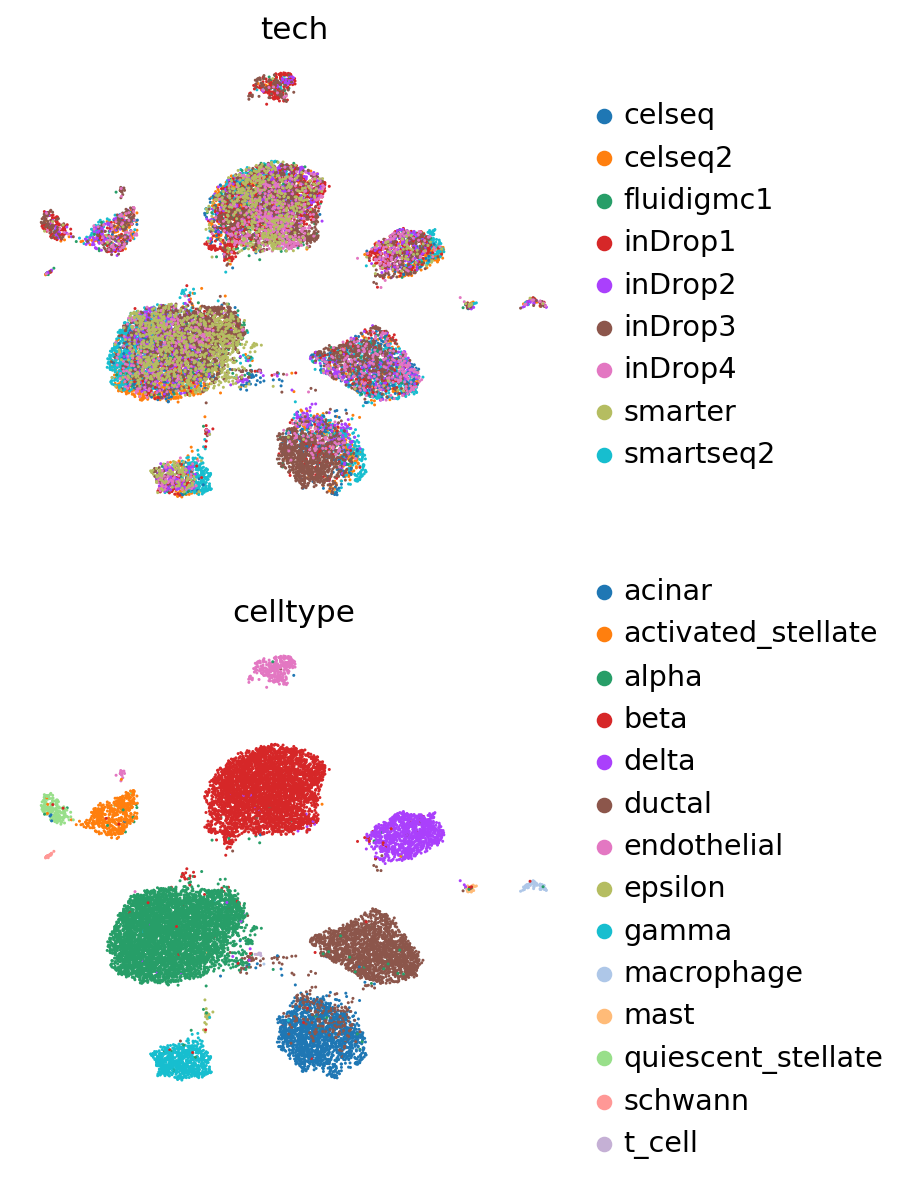
[37]:
ax = sc.pl.umap(
adata_full,
frameon=False,
show=False,
)
sc.pl.umap(
adata_full[: adata_query.n_obs],
color=["predictions"],
frameon=False,
title="Query predictions",
ax=ax,
alpha=0.7,
)
ax = sc.pl.umap(
adata_full,
frameon=False,
show=False,
)
sc.pl.umap(
adata_full[: adata_query.n_obs],
color=["celltype"],
frameon=False,
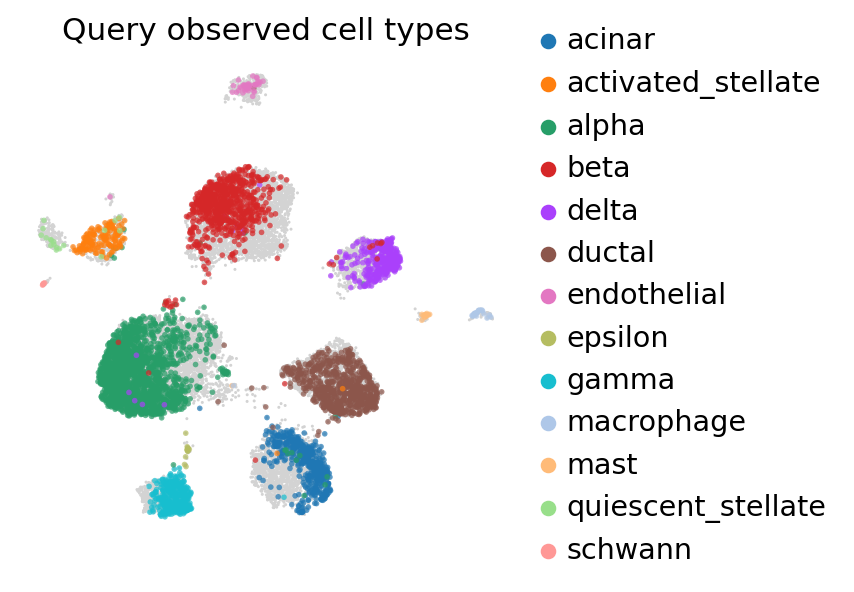
title="Query observed cell types",
ax=ax,
alpha=0.7,
)
/usr/local/lib/python3.7/dist-packages/anndata/compat/_overloaded_dict.py:106: ImplicitModificationWarning: Trying to modify attribute `._uns` of view, initializing view as actual.
self.data[key] = value
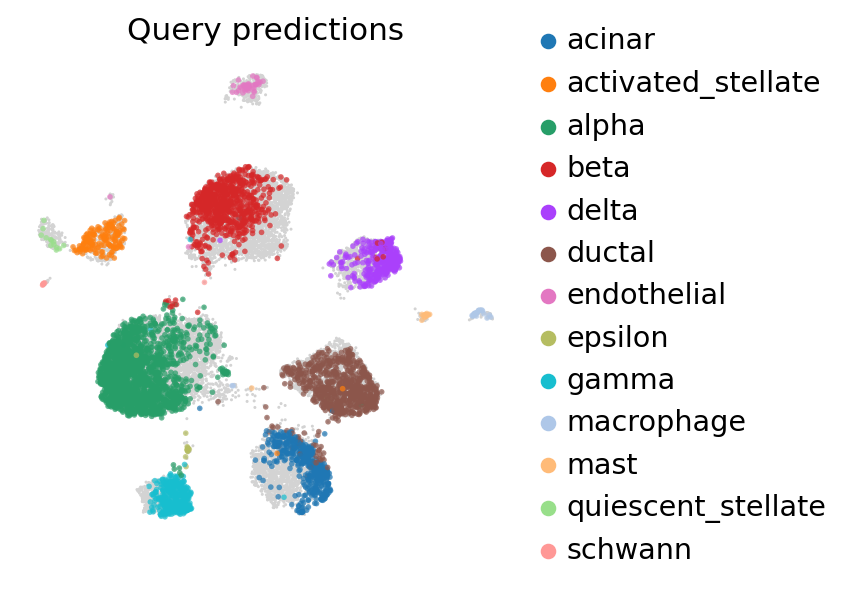
Reference mapping with TOTALVI#
This workflow works very similarly for TOTALVI. Here we demonstrate how to build a CITE-seq reference and use scRNA-seq only data as the query.
Assemble data#
For totalVI, we will treat two CITE-seq PBMC datasets from 10X Genomics as the reference. These datasets were already filtered for outliers like doublets, as described in the totalVI manuscript. There are 14 proteins in the reference.
[38]:
adata_ref = scvi.data.pbmcs_10x_cite_seq()
INFO Downloading file at data/pbmc_10k_protein_v3.h5ad
Downloading...: 24938it [00:00, 119159.97it/s]
INFO Downloading file at data/pbmc_5k_protein_v3.h5ad
Downloading...: 100%|██████████| 18295/18295.0 [00:00<00:00, 109289.36it/s]
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
In general, there will be some necessary data wrangling. For example, we need to provide totalVI with some protein data – and when it’s all zeros, totalVI identifies that the protein data is missing in this “batch”.
It could have also been the case that only some of the protein data was missing, in which case we would add zeros for each of the missing proteins.
[39]:
adata_query = scvi.data.dataset_10x("pbmc_10k_v3")
adata_query.obs["batch"] = "PBMC 10k (RNA only)"
# put matrix of zeros for protein expression (considered missing)
pro_exp = adata_ref.obsm["protein_expression"]
data = np.zeros((adata_query.n_obs, pro_exp.shape[1]))
adata_query.obsm["protein_expression"] = pd.DataFrame(
columns=pro_exp.columns, index=adata_query.obs_names, data=data
)
INFO Downloading file at data/10X/pbmc_10k_v3/filtered_feature_bc_matrix.h5
Downloading...: 37492it [00:02, 18127.05it/s]
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1830: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
We do some light QC filtering on the query dataset (doublets, mitochondrial, etc.)
[40]:
scrub = scr.Scrublet(adata_query.X)
doublet_scores, predicted_doublets = scrub.scrub_doublets()
adata_query = adata_query[~predicted_doublets].copy()
adata_query.var["mt"] = adata_query.var_names.str.startswith(
"MT-"
) # annotate the group of mitochondrial genes as 'mt'
sc.pp.calculate_qc_metrics(
adata_query, qc_vars=["mt"], percent_top=None, log1p=False, inplace=True
)
adata_query = adata_query[adata_query.obs.pct_counts_mt < 15, :].copy()
Preprocessing...
Simulating doublets...
Embedding transcriptomes using PCA...
Calculating doublet scores...
Automatically set threshold at doublet score = 0.33
Detected doublet rate = 4.7%
Estimated detectable doublet fraction = 55.3%
Overall doublet rate:
Expected = 10.0%
Estimated = 8.6%
Elapsed time: 16.2 seconds
Now to concatenate the objects, which intersects the genes properly.
[41]:
adata_full = anndata.concat([adata_ref, adata_query])
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
And split them back up into reference and query (but now genes are properly aligned between objects).
[42]:
adata_ref = adata_full[
np.logical_or(adata_full.obs.batch == "PBMC5k", adata_full.obs.batch == "PBMC10k")
].copy()
adata_query = adata_full[adata_full.obs.batch == "PBMC 10k (RNA only)"].copy()
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
We run gene selection on the reference, because that’s all that will be avaialble to us at first.
[43]:
sc.pp.highly_variable_genes(
adata_ref,
n_top_genes=4000,
flavor="seurat_v3",
batch_key="batch",
subset=True,
)
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
Finally, we use these selected genes for the query dataset as well.
[44]:
adata_query = adata_query[:, adata_ref.var_names].copy()
Train reference#
[45]:
scvi.model.TOTALVI.setup_anndata(
adata_ref, batch_key="batch", protein_expression_obsm_key="protein_expression"
)
INFO Using column names from columns of adata.obsm['protein_expression']
[46]:
arches_params = dict(
use_layer_norm="both",
use_batch_norm="none",
)
vae_ref = scvi.model.TOTALVI(adata_ref, **arches_params)
INFO Computing empirical prior initialization for protein background.
[47]:
vae_ref.train()
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
/usr/local/lib/python3.7/dist-packages/pytorch_lightning/trainer/connectors/data_connector.py:489: PossibleUserWarning: Your `val_dataloader`'s sampler has shuffling enabled, it is strongly recommended that you turn shuffling off for val/test/predict dataloaders.
category=PossibleUserWarning,
Epoch 309/400: 77%|███████▋ | 309/400 [04:48<01:24, 1.08it/s, loss=1.23e+03, v_num=1]Epoch 309: reducing learning rate of group 0 to 2.4000e-03.
Epoch 358/400: 90%|████████▉ | 358/400 [05:34<00:38, 1.08it/s, loss=1.22e+03, v_num=1]Epoch 358: reducing learning rate of group 0 to 1.4400e-03.
Epoch 400/400: 100%|██████████| 400/400 [06:13<00:00, 1.07it/s, loss=1.22e+03, v_num=1]
[48]:
adata_ref.obsm["X_totalVI"] = vae_ref.get_latent_representation()
sc.pp.neighbors(adata_ref, use_rep="X_totalVI")
sc.tl.umap(adata_ref, min_dist=0.4)
[49]:
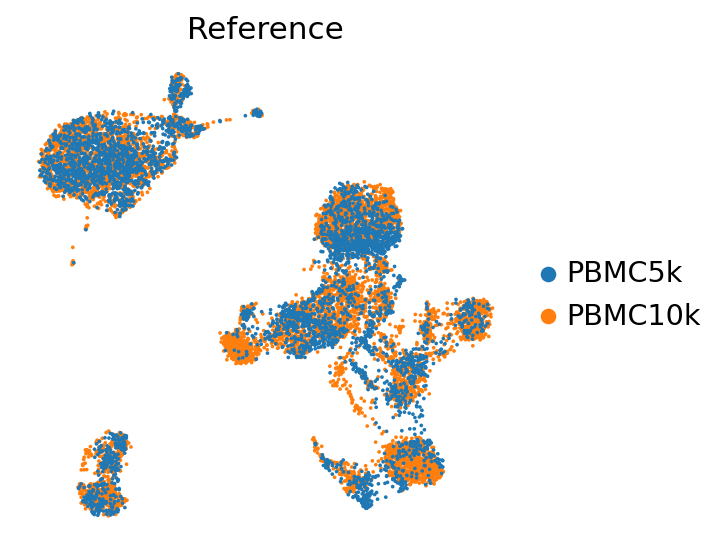
sc.pl.umap(adata_ref, color=["batch"], frameon=False, ncols=1, title="Reference")
[50]:
dir_path = "saved_model/"
vae_ref.save(dir_path, overwrite=True)
Update with query#
[51]:
scvi.model.TOTALVI.prepare_query_anndata(adata_query, dir_path)
vae_q = scvi.model.TOTALVI.load_query_data(
adata_query,
dir_path,
)
INFO File saved_model/model.pt already downloaded
INFO Found 100.0% reference vars in query data.
INFO File saved_model/model.pt already downloaded
INFO Found batches with missing protein expression
INFO Computing empirical prior initialization for protein background.
/usr/local/lib/python3.7/dist-packages/scvi/model/_totalvi.py:133: UserWarning: Some proteins have all 0 counts in some batches. These proteins will be treated as missing measurements; however, this can occur due to experimental design/biology. Reinitialize the model with `override_missing_proteins=True`,to override this behavior.
warnings.warn(msg, UserWarning)
[52]:
vae_q.train(200, plan_kwargs=dict(weight_decay=0.0))
GPU available: True, used: True
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
LOCAL_RANK: 0 - CUDA_VISIBLE_DEVICES: [0]
/usr/local/lib/python3.7/dist-packages/pytorch_lightning/trainer/connectors/data_connector.py:489: PossibleUserWarning: Your `val_dataloader`'s sampler has shuffling enabled, it is strongly recommended that you turn shuffling off for val/test/predict dataloaders.
category=PossibleUserWarning,
Epoch 200/200: 100%|██████████| 200/200 [03:43<00:00, 1.12s/it, loss=759, v_num=1]
[53]:
adata_query.obsm["X_totalVI"] = vae_q.get_latent_representation()
sc.pp.neighbors(adata_query, use_rep="X_totalVI")
sc.tl.umap(adata_query, min_dist=0.4)
Impute protein data for query and visualize#
Now that we have updated with the query, we can impute the proteins that were observed in the reference, using the transform_batch parameter.
[54]:
_, imputed_proteins = vae_q.get_normalized_expression(
adata_query,
n_samples=10,
return_mean=True,
transform_batch=["PBMC10k", "PBMC5k"],
)
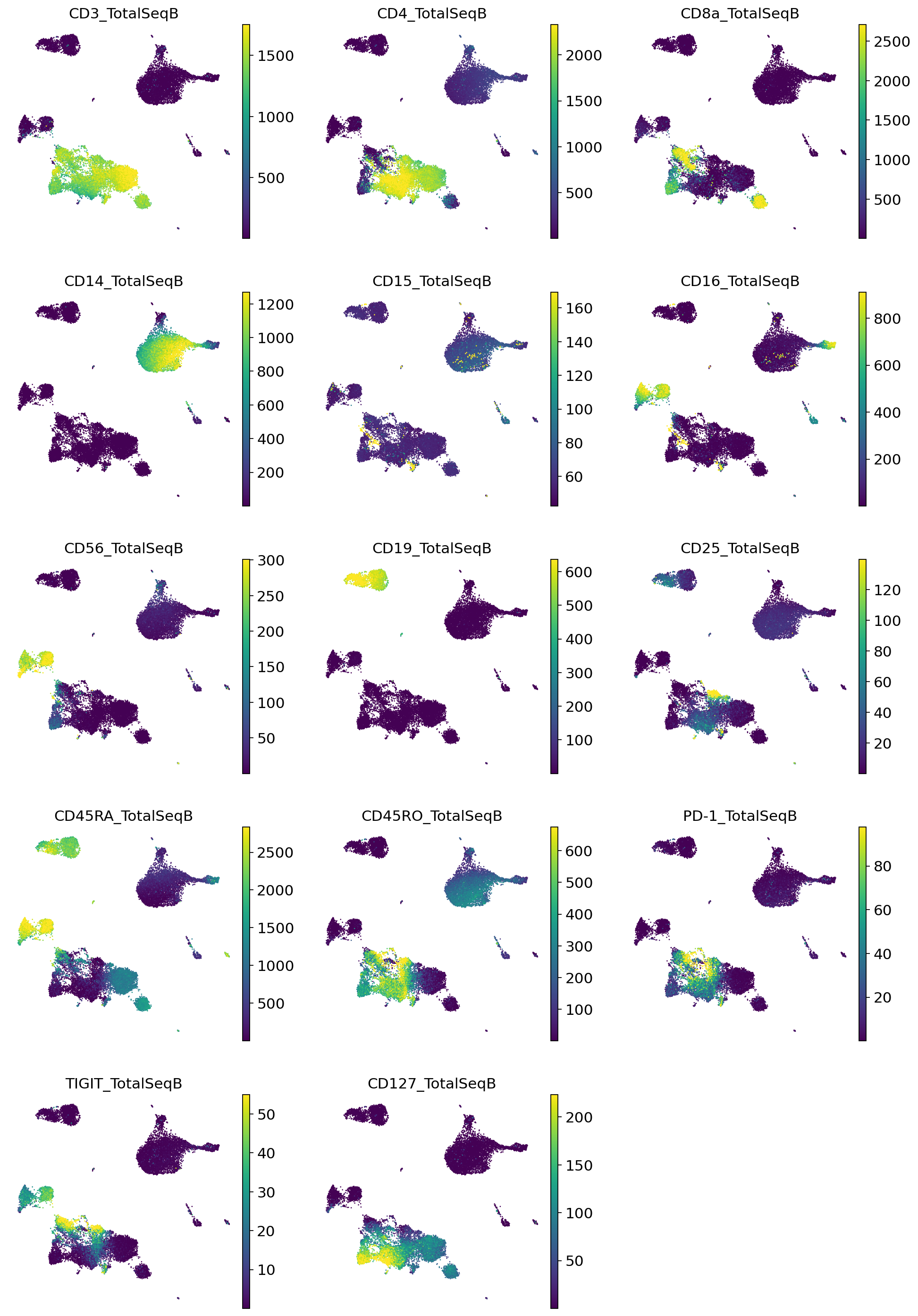
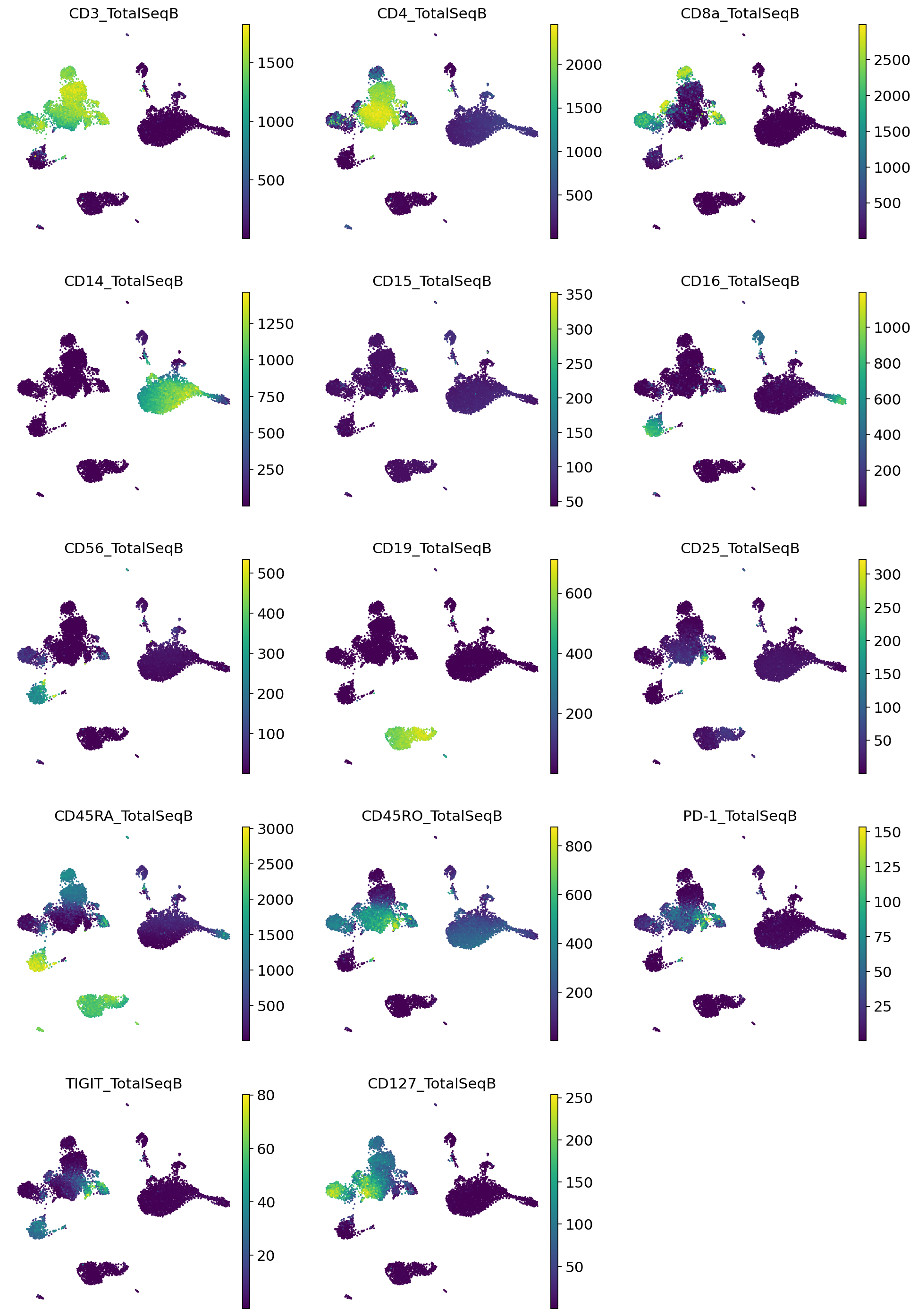
Very quickly we can identify the major expected subpopulations of B cells, CD4 T cells, CD8 T cells, monocytes, etc.
[55]:
adata_query.obs = pd.concat([adata_query.obs, imputed_proteins], axis=1)
sc.pl.umap(
adata_query,
color=imputed_proteins.columns,
frameon=False,
ncols=3,
)
Visualize reference and query#
[56]:
adata_full_new = adata_query.concatenate(adata_ref, batch_key="none")
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
[57]:
adata_full_new.obsm["X_totalVI"] = vae_q.get_latent_representation(adata_full_new)
sc.pp.neighbors(adata_full_new, use_rep="X_totalVI")
sc.tl.umap(adata_full_new, min_dist=0.3)
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
INFO Found batches with missing protein expression
[58]:
_, imputed_proteins_all = vae_q.get_normalized_expression(
adata_full_new,
n_samples=10,
return_mean=True,
transform_batch=["PBMC10k", "PBMC5k"],
)
for i, p in enumerate(imputed_proteins_all.columns):
adata_full_new.obs[p] = imputed_proteins_all[p].to_numpy().copy()
[59]:
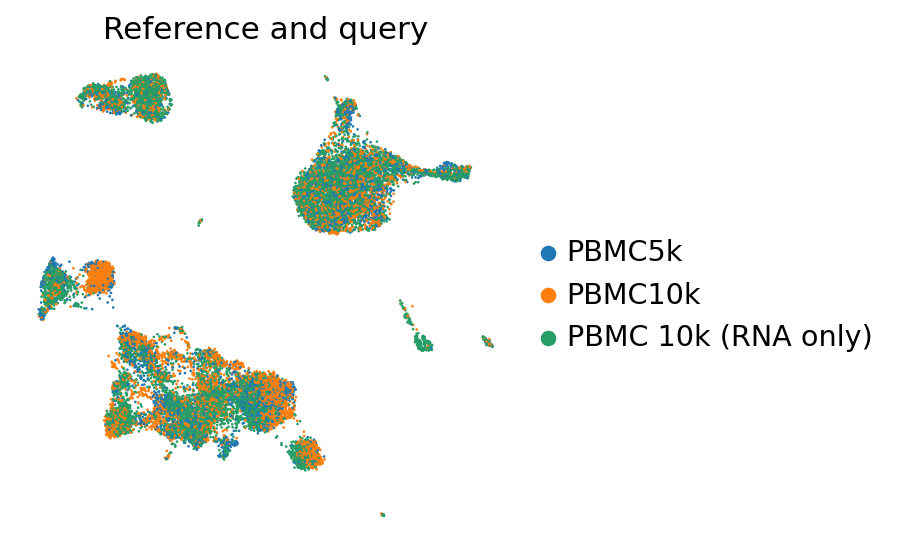
perm_inds = np.random.permutation(np.arange(adata_full_new.n_obs))
sc.pl.umap(
adata_full_new[perm_inds],
color=["batch"],
frameon=False,
ncols=1,
title="Reference and query",
)
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1235: ImplicitModificationWarning: Trying to modify attribute `.obs` of view, initializing view as actual.
df[key] = c
/usr/local/lib/python3.7/dist-packages/anndata/_core/anndata.py:1828: UserWarning: Observation names are not unique. To make them unique, call `.obs_names_make_unique`.
utils.warn_names_duplicates("obs")
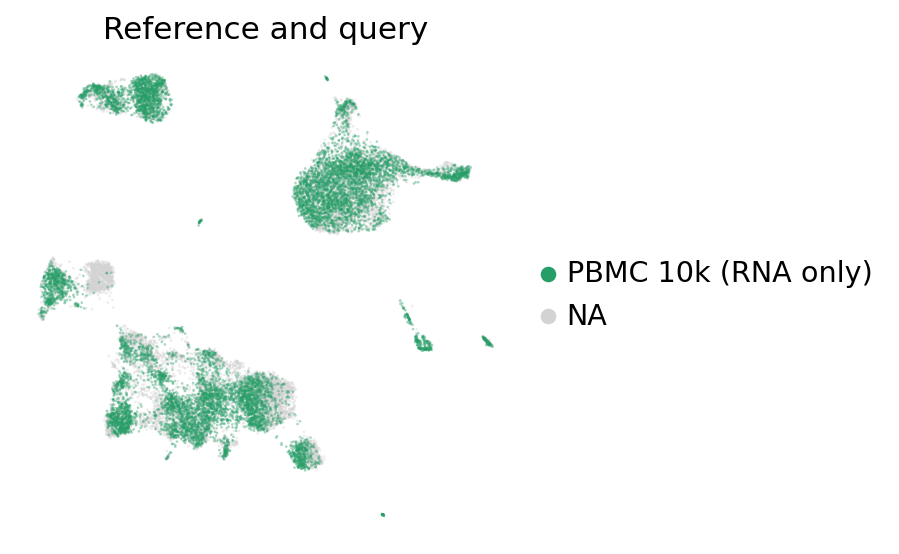
[60]:
ax = sc.pl.umap(
adata_full_new,
color="batch",
groups=["PBMC 10k (RNA only)"],
frameon=False,
ncols=1,
title="Reference and query",
alpha=0.4,
)
[61]:
sc.pl.umap(
adata_full_new,
color=imputed_proteins_all.columns,
frameon=False,
ncols=3,
vmax="p99",
)