6. Clustering 3K PBMCs with scVI and ScanPy¶
Disclaimer: some of the code in this notebook was taken from Scanpy’s Clustering tutorial (https://scanpy-tutorials.readthedocs.io/en/latest/pbmc3k.html) which is itself based on SEURAT’s clustering tutorial in R.
This notebook is designed as a demonstration of scVI’s potency on the tasks considered in the Scanpy PBMC 3K Clustering notebook. In order to do so, we follow the same workflow adopted by scanpy in their clustering tutorial while performing the analysis using scVI as often as possible. Specifically, we use scVI’s latent representation and differential expression analysis (which computes a Bayes Factor on imputed values). For visualisation, pre-processing and for some canonical analysis, we use the Scanpy package directly.
When useful, we provide high-level wrappers around scVI’s analysis tools. These functions are designed to make standard use of scVI as easy as possible. For specific use cases, we encourage the reader to take a closer look at those functions and modify them according to their needs.
[ ]:
# If running in Colab, navigate to Runtime -> Change runtime type
# and ensure you're using a Python 3 runtime with GPU hardware accelerator
# installation in Colab can take several minutes
6.1. Colab and automated testing configuration¶
[1]:
def allow_notebook_for_test():
print("Testing the scanpy pbmc3k notebook")
import sys, os
n_epochs_all = None
test_mode = False
show_plot = True
def if_not_test_else(x, y):
if not test_mode:
return x
else:
return y
IN_COLAB = "google.colab" in sys.modules
show_plot = True
test_mode = False
n_epochs_all = None
save_path = "data/"
if not test_mode and not IN_COLAB:
save_path = "../../data"
if IN_COLAB:
!pip install --quiet scvi[notebooks]
6.2. Initialization¶
[2]:
download_data = False
if IN_COLAB or download_data:
!mkdir data
!wget http://cf.10xgenomics.com/samples/cell-exp/1.1.0/pbmc3k/pbmc3k_filtered_gene_bc_matrices.tar.gz -O data/pbmc3k_filtered_gene_bc_matrices.tar.gz
!cd data; tar -xzf pbmc3k_filtered_gene_bc_matrices.tar.gz
[3]:
# Seed for reproducibility
import torch
import numpy as np
import pandas as pd
import scanpy as sc
from typing import Tuple
# scVI imports
import scvi
from scvi.dataset import AnnDatasetFromAnnData
from scvi.inference import UnsupervisedTrainer
from scvi.models.vae import VAE
torch.manual_seed(0)
np.random.seed(0)
sc.settings.verbosity = 0 # verbosity: errors (0), warnings (1), info (2), hints (3)
[5]:
if not test_mode:
sc.settings.set_figure_params(dpi=60)
%matplotlib inline
[6]:
# Load the data
adata = sc.read_10x_mtx(
os.path.join(
save_path, "filtered_gene_bc_matrices/hg19/"
), # the directory with the `.mtx` file
var_names="gene_symbols", # use gene symbols for the variable names (variables-axis index)
)
adata.var_names_make_unique()
6.3. Preprocessing¶
In the following section, we reproduce the preprocessing steps adopted in the scanpy notebook.
Basic filtering: we remove cells with a low number of genes expressed and genes which are expressed in a low number of cells.
[7]:
min_genes = if_not_test_else(200, 0)
min_cells = if_not_test_else(3, 0)
[8]:
sc.settings.verbosity = 2
sc.pp.filter_cells(adata, min_genes=min_genes)
sc.pp.filter_genes(adata, min_cells=min_cells)
sc.pp.filter_cells(adata, min_genes=1)
filtered out 19024 genes that are detected in less than 3 cells
As in the scanpy notebook, we then look for high levels of mitochondrial genes and high number of expressed genes which are indicators of poor quality cells.
[9]:
mito_genes = adata.var_names.str.startswith("MT-")
adata.obs["percent_mito"] = (
np.sum(adata[:, mito_genes].X, axis=1).A1 / np.sum(adata.X, axis=1).A1
)
adata.obs["n_counts"] = adata.X.sum(axis=1).A1
[10]:
adata = adata[adata.obs["n_genes"] < 2500, :]
adata = adata[adata.obs["percent_mito"] < 0.05, :]
6.4. ⚠ scVI uses non normalized data so we keep the original data in a separate AnnData object, then the normalization steps are performed¶
6.4.1. Normalization and more filtering¶
We only keep highly variable genes
[11]:
adata_original = adata.copy()
sc.pp.normalize_per_cell(adata, counts_per_cell_after=1e4)
sc.pp.log1p(adata)
min_mean = if_not_test_else(0.0125, -np.inf)
max_mean = if_not_test_else(3, np.inf)
min_disp = if_not_test_else(0.5, -np.inf)
max_disp = if_not_test_else(None, np.inf)
sc.pp.highly_variable_genes(
adata,
min_mean=min_mean,
max_mean=max_mean,
min_disp=min_disp,
max_disp=max_disp
)
highly_variable_genes = adata.var["highly_variable"]
adata = adata[:, highly_variable_genes]
adata.raw = adata
sc.pp.regress_out(adata, ["n_counts", "percent_mito"])
sc.pp.scale(adata, max_value=10)
# Also filter the original adata genes
adata_original = adata_original[:, highly_variable_genes]
print("{} highly variable genes".format(highly_variable_genes.sum()))
regressing out ['n_counts', 'percent_mito']
sparse input is densified and may lead to high memory use
finished (0:00:12.37)
1838
6.5. Compute the scVI latent space¶
Below we provide then use a wrapper function designed to compute scVI’s latent representation of the non-normalized data. Specifically, we train scVI’s VAE, compute and store the latent representation then return the posterior which will later be used for further inference.
[12]:
def compute_scvi_latent(
adata: sc.AnnData,
n_latent: int = 5,
n_epochs: int = 100,
lr: float = 1e-3,
use_batches: bool = False,
use_cuda: bool = False,
) -> Tuple[scvi.inference.Posterior, np.ndarray]:
"""Train and return a scVI model and sample a latent space
:param adata: sc.AnnData object non-normalized
:param n_latent: dimension of the latent space
:param n_epochs: number of training epochs
:param lr: learning rate
:param use_batches
:param use_cuda
:return: (scvi.Posterior, latent_space)
"""
# Convert easily to scvi dataset
scviDataset = AnnDatasetFromAnnData(adata)
# Train a model
vae = VAE(
scviDataset.nb_genes,
n_batch=scviDataset.n_batches * use_batches,
n_latent=n_latent,
)
trainer = UnsupervisedTrainer(vae, scviDataset, train_size=1.0, use_cuda=use_cuda)
trainer.train(n_epochs=n_epochs, lr=lr)
####
# Extract latent space
posterior = trainer.create_posterior(
trainer.model, scviDataset, indices=np.arange(len(scviDataset))
).sequential()
latent, _, _ = posterior.get_latent()
return posterior, latent
[13]:
n_epochs = 400 if n_epochs_all is None else n_epochs_all
# use_cuda to use GPU
use_cuda = True if IN_COLAB else False
scvi_posterior, scvi_latent = compute_scvi_latent(
adata_original, n_epochs=n_epochs, n_latent=10, use_cuda=use_cuda
)
adata.obsm["X_scvi"] = scvi_latent
# store scvi imputed expression
scale = scvi_posterior.get_sample_scale()
for _ in range(9):
scale += scvi_posterior.get_sample_scale()
scale /= 10
for gene, gene_scale in zip(adata.var.index, np.squeeze(scale).T):
adata.obs["scale_" + gene] = gene_scale
training: 100%|██████████| 10/10 [00:32<00:00, 3.21s/it]
6.6. Principal component analysis to reproduce ScanPy results and compare them against scVI’s¶
Below, we reproduce exactly scanpy’s PCA on normalized data.
[14]:
sc.tl.pca(adata, svd_solver="arpack")
[15]:
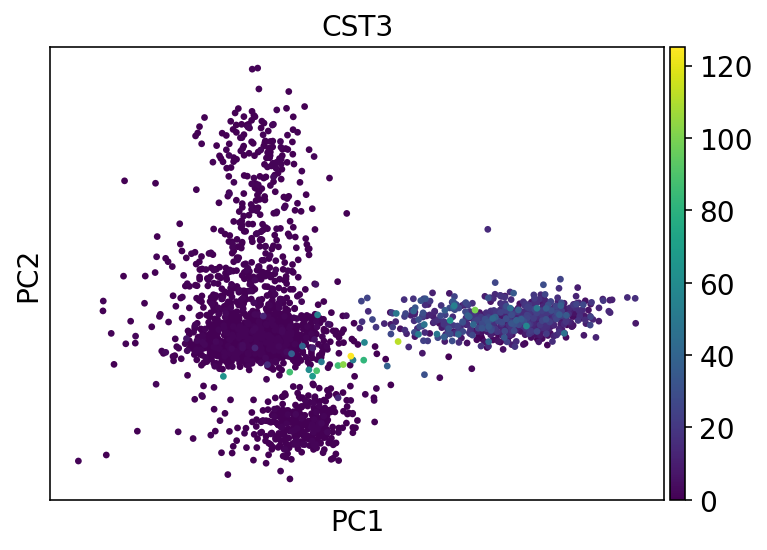
sc.pl.pca(adata, color="CST3", show=show_plot)
[16]:
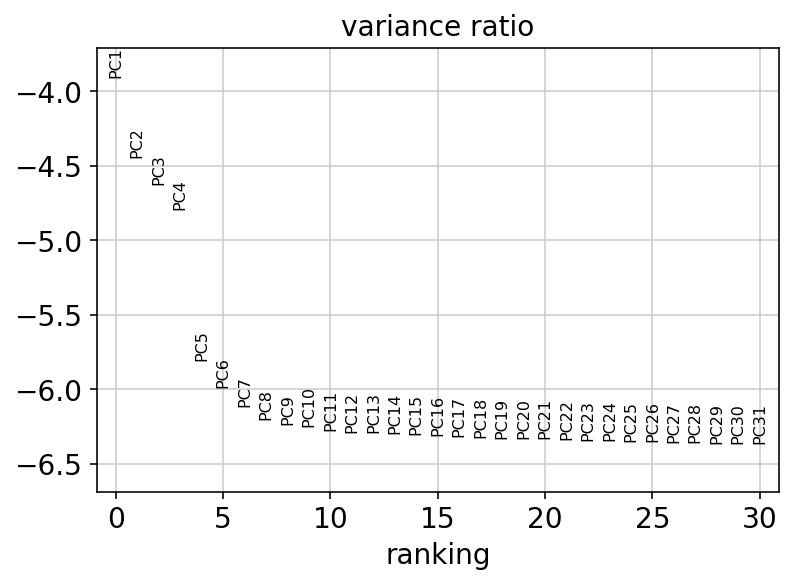
sc.pl.pca_variance_ratio(adata, log=True, show=show_plot)
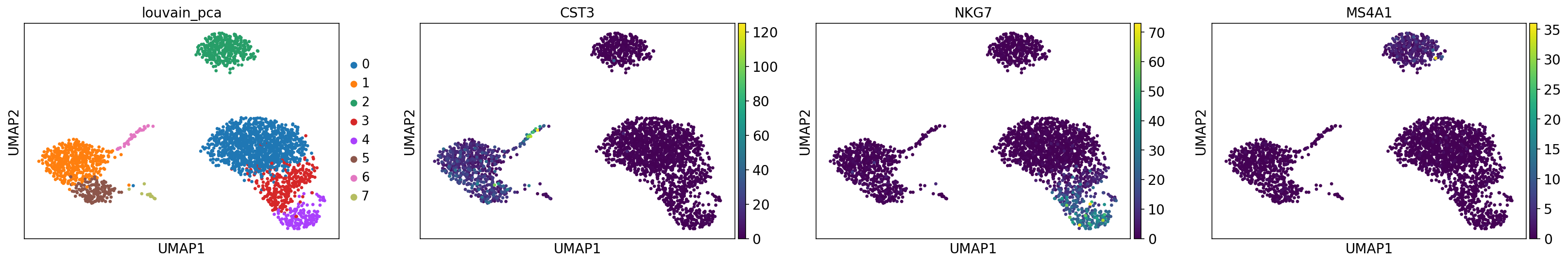
6.7. Computing, embedding and clustering the neighborhood graph¶
The Scanpy API computes a neighborhood graph with sc.pp.neighbors which can be called to work on a specific representation use_rep='your rep'. Once the neighbors graph has been computed, all Scanpy algorithms working on it can be called as usual (that is louvain, paga, umap …)
[17]:
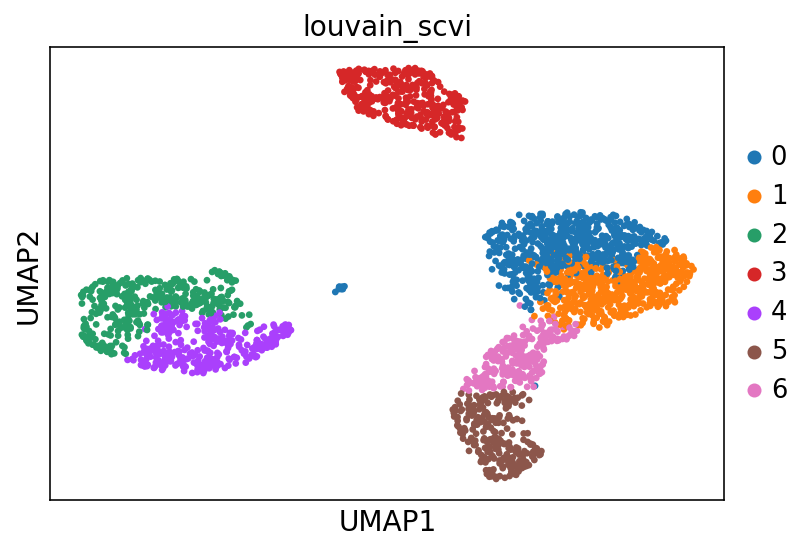
sc.pp.neighbors(adata, n_neighbors=10, n_pcs=40)
sc.tl.leiden(adata, key_added="leiden_pca")
sc.tl.umap(adata)
computing neighbors
using 'X_pca' with n_pcs = 40
finished (0:00:06.70)
running Louvain clustering
using the "louvain" package of Traag (2017)
finished (0:00:00.33)
computing UMAP
finished (0:00:13.68)
[18]:
sc.pl.umap(adata, color=["leiden_pca", "CST3", "NKG7", "MS4A1"], ncols=4, show=show_plot)
[19]:
sc.pp.neighbors(adata, n_neighbors=20, n_pcs=40, use_rep="X_scvi")
sc.tl.umap(adata)
computing neighbors
finished (0:00:01.14)
computing UMAP
finished (0:00:12.35)
[20]:
sc.tl.leiden(adata, key_added="leiden_scvi", resolution=0.8)
running Louvain clustering
using the "louvain" package of Traag (2017)
finished (0:00:00.53)
[21]:
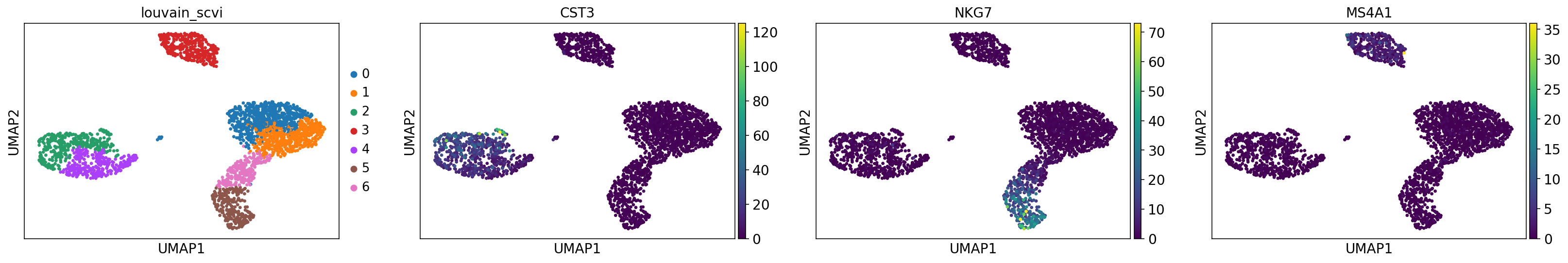
# use scVI imputed values for plotting
sc.pl.umap(adata, color=["leiden_scvi", "scale_CST3", "scale_NKG7", "scale_MS4A1"], ncols=4, show=show_plot)
6.8. Finding marker genes¶
ScanPy tries to determine marker genes using a t-test and a Wilcoxon test.
For the same task, from scVI’s trained VAE model we can sample the gene expression rate for each gene in each cell. For the two populations of interest, we can then randomly sample pairs of cells, one from each population to compare their expression rate for a gene. The degree of differential expression is measured by logit(\(\frac{p}{1-p}\)) (Bayes Factor) where \(p\) is the probability of a cell from population \(A\) having a higher expression than a cell from population \(B\) (in “change” mode higher expression refers to the log fold change of expression being greater than \(\delta = 0.5\)).
Below, we provide a wrapper around scVI’s differential expression process. Specifically, it computes the average of the Bayes factor where population \(A\) covers each cluster in adata.obs[label_name] and is compared with the aggregate formed by all the other clusters.
[22]:
def rank_genes_groups_bayes(
adata: sc.AnnData,
scvi_posterior: scvi.inference.Posterior,
n_samples: int = 5000,
M_permutation: int = 10000,
n_genes: int = 25,
label_name: str = "leiden_scvi",
mode: str = "vanilla"
) -> pd.DataFrame:
"""
Rank genes for characterizing groups.
Computes Bayes factor for each cluster against the others to test for differential expression.
See Nature article (https://rdcu.be/bdHYQ)
:param adata: sc.AnnData object non-normalized
:param scvi_posterior:
:param n_samples:
:param M_permutation:
:param n_genes:
:param label_name: The groups tested are taken from adata.obs[label_name] which can be computed
using clustering like Louvain (Ex: sc.tl.louvain(adata, key_added=label_name) )
:return: Summary of Bayes factor per gene, per cluster
"""
# Call scvi function
per_cluster_de, cluster_id = scvi_posterior.one_vs_all_degenes(
cell_labels=np.asarray(adata.obs[label_name].values).astype(int).ravel(),
min_cells=1,
n_samples=n_samples,
M_permutation=M_permutation,
mode=mode
)
# convert to ScanPy format -- this is just about feeding scvi results into a format readable by ScanPy
markers = []
scores = []
names = []
for i, x in enumerate(per_cluster_de):
subset_de = x[:n_genes]
markers.append(subset_de)
scores.append(tuple(subset_de["bayes_factor"].values))
names.append(tuple(subset_de.index.values))
markers = pd.concat(markers)
dtypes_scores = [(str(i), "<f4") for i in range(len(scores))]
dtypes_names = [(str(i), "<U50") for i in range(len(names))]
scores = np.array([tuple(row) for row in np.array(scores).T], dtype=dtypes_scores)
scores = scores.view(np.recarray)
names = np.array([tuple(row) for row in np.array(names).T], dtype=dtypes_names)
names = names.view(np.recarray)
adata.uns["rank_genes_groups_scvi"] = {
"params": {
"groupby": "",
"reference": "rest",
"method": "",
"use_raw": True,
"corr_method": "",
},
"scores": scores,
"names": names,
}
return markers
[23]:
n_genes = 20
sc.tl.rank_genes_groups(
adata,
"leiden_scvi",
method="t-test",
use_raw=True,
key_added="rank_genes_groups_ttest",
n_genes=n_genes,
)
sc.tl.rank_genes_groups(
adata,
"leiden_scvi",
method="wilcoxon",
use_raw=True,
key_added="rank_genes_groups_wilcox",
n_genes=n_genes,
)
sc.pl.rank_genes_groups(
adata, key="rank_genes_groups_ttest", sharey=False, n_genes=n_genes, show=show_plot
)
sc.pl.rank_genes_groups(
adata, key="rank_genes_groups_wilcox", sharey=False, n_genes=n_genes, show=show_plot
)
ranking genes
finished (0:00:00.65)
ranking genes
finished (0:00:01.49)
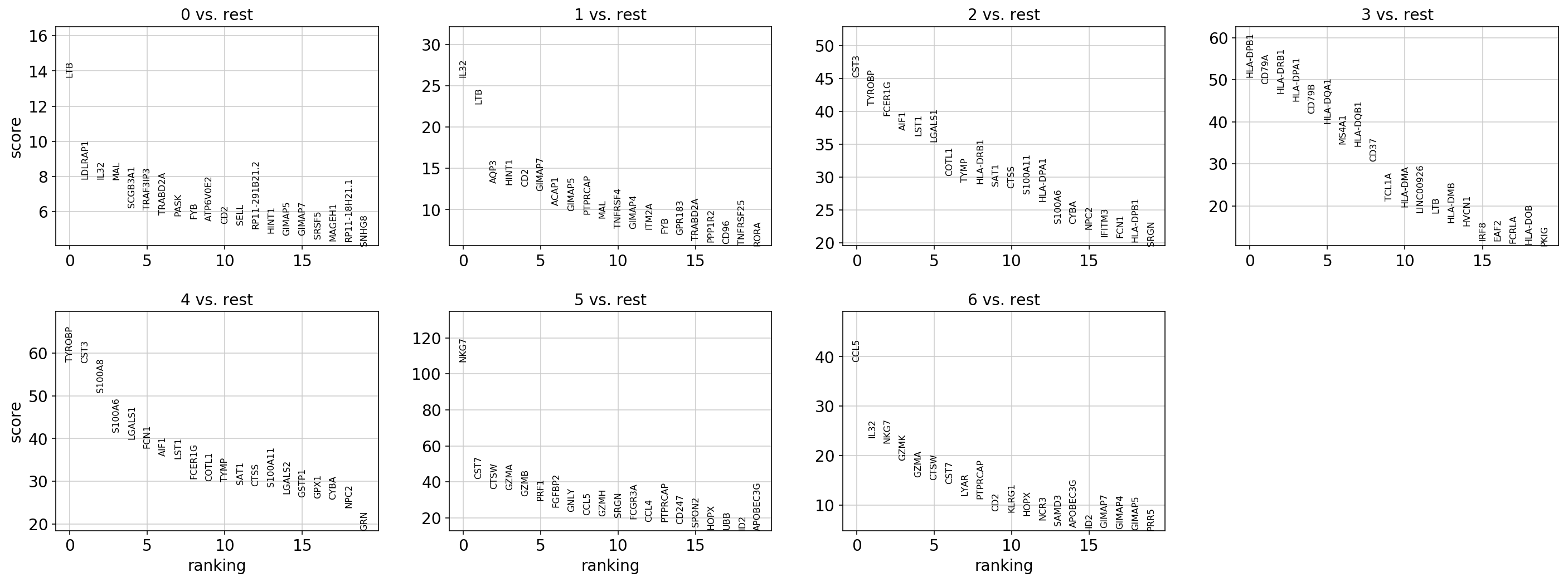
[24]:
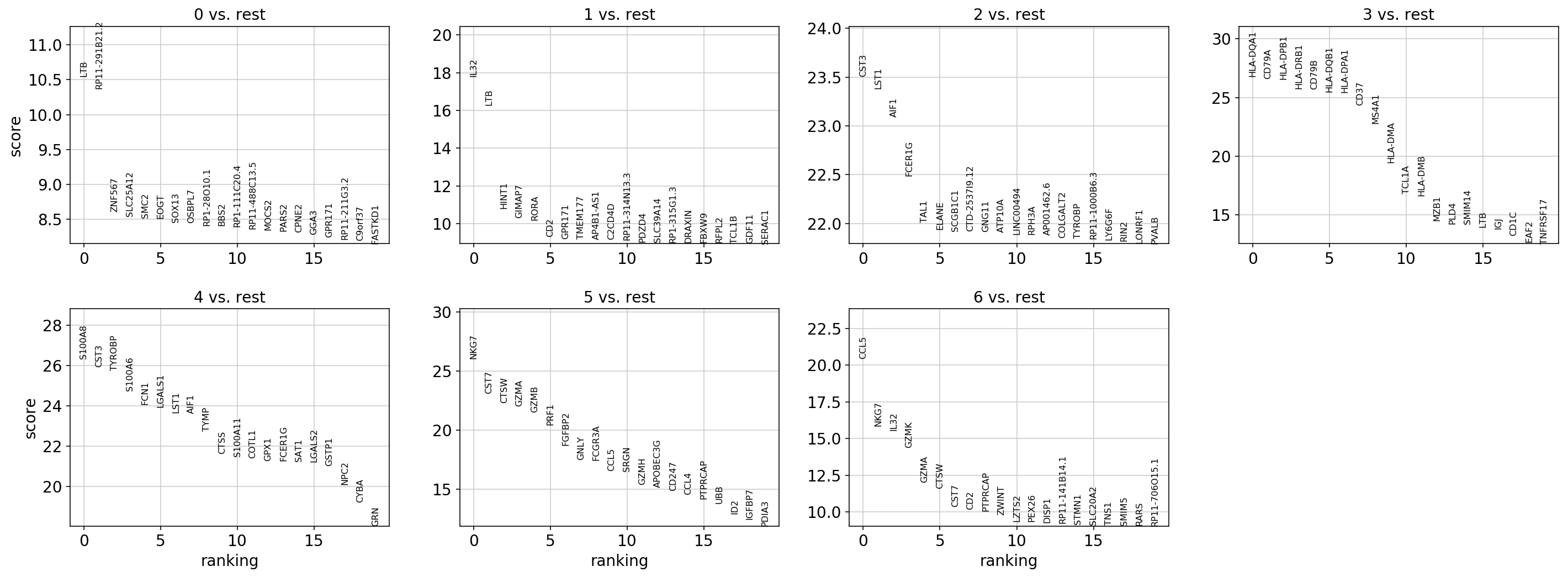
rank_genes_groups_bayes(
adata, scvi_posterior, label_name="leiden_scvi", n_genes=n_genes
)
sc.pl.rank_genes_groups(
adata, key="rank_genes_groups_scvi", sharey=False, n_genes=n_genes, show=show_plot
)
[25]:
# We compute the rank of every gene to perform analysis after
all_genes = len(adata.var_names)
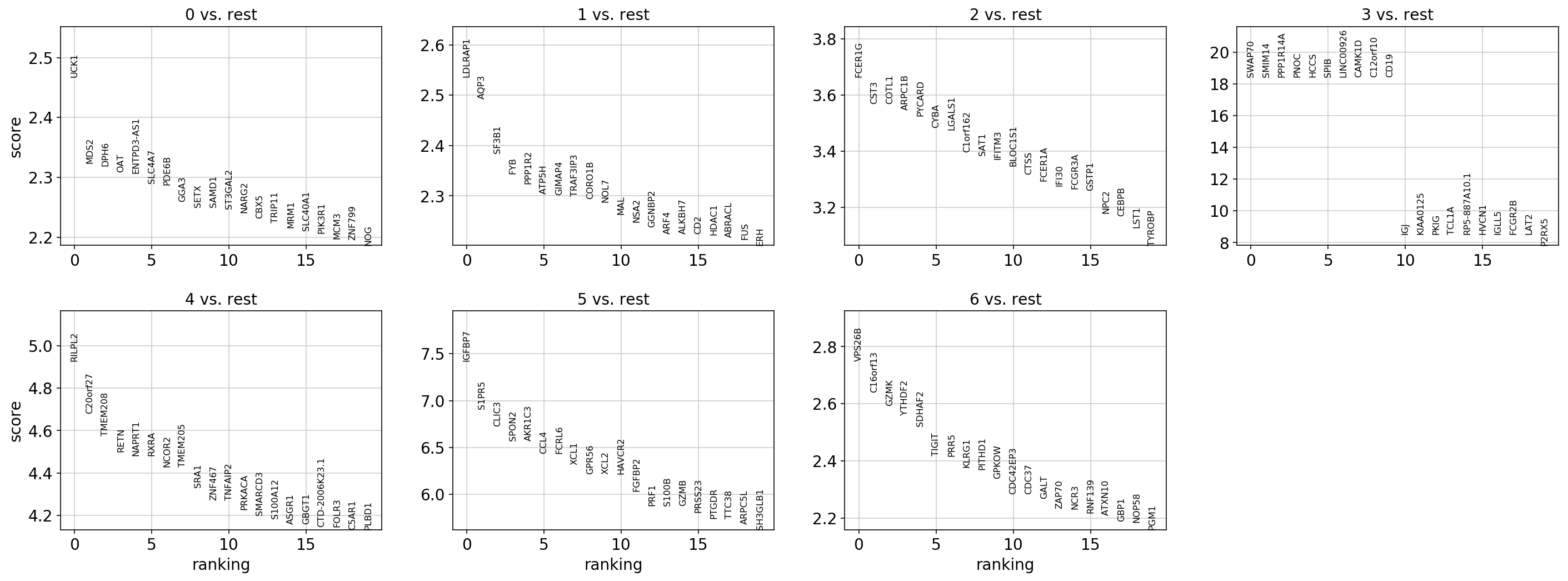
sc.tl.rank_genes_groups(adata, 'leiden_scvi', method='t-test', use_raw=False, key_added='rank_genes_groups_ttest', n_genes=all_genes)
sc.tl.rank_genes_groups(adata, 'leiden_scvi', method='wilcoxon', use_raw=False, key_added='rank_genes_groups_wilcox', n_genes=all_genes)
differential_expression = rank_genes_groups_bayes(adata, scvi_posterior, label_name='leiden_scvi', n_genes=all_genes)
ranking genes
finished (0:00:00.67)
ranking genes
finished (0:00:01.50)
[26]:
def ratio(A, B):
A, B = set(A), set(B)
return len(A.intersection(B)) / len(A) * 100
[27]:
cluster_distrib = adata.obs.groupby("leiden_scvi").count()["n_genes"]
For each cluster, we compute the percentage of genes which are in the n_genes most expressed genes of both Scanpy’s and scVI’s differential expression tests.
[28]:
n_genes = 25
sc.pl.umap(adata, color=["leiden_scvi"], ncols=1, show=show_plot)
for c in cluster_distrib.index:
print(
"Cluster %s (%d cells): t-test / wilcox %6.2f %% & t-test / scvi %6.2f %%"
% (
c,
cluster_distrib[c],
ratio(
adata.uns["rank_genes_groups_ttest"]["names"][c][:n_genes],
adata.uns["rank_genes_groups_wilcox"]["names"][c][:n_genes],
),
ratio(
adata.uns["rank_genes_groups_ttest"]["names"][c][:n_genes],
adata.uns["rank_genes_groups_scvi"]["names"][c][:n_genes],
),
)
)
Cluster 0 (613 cells): t-test / wilcox 8.00 % & t-test / scvi 4.00 %
Cluster 1 (526 cells): t-test / wilcox 28.00 % & t-test / scvi 40.00 %
Cluster 2 (355 cells): t-test / wilcox 20.00 % & t-test / scvi 76.00 %
Cluster 3 (345 cells): t-test / wilcox 64.00 % & t-test / scvi 40.00 %
Cluster 4 (317 cells): t-test / wilcox 100.00 % & t-test / scvi 0.00 %
Cluster 5 (254 cells): t-test / wilcox 76.00 % & t-test / scvi 40.00 %
Cluster 6 (228 cells): t-test / wilcox 36.00 % & t-test / scvi 24.00 %
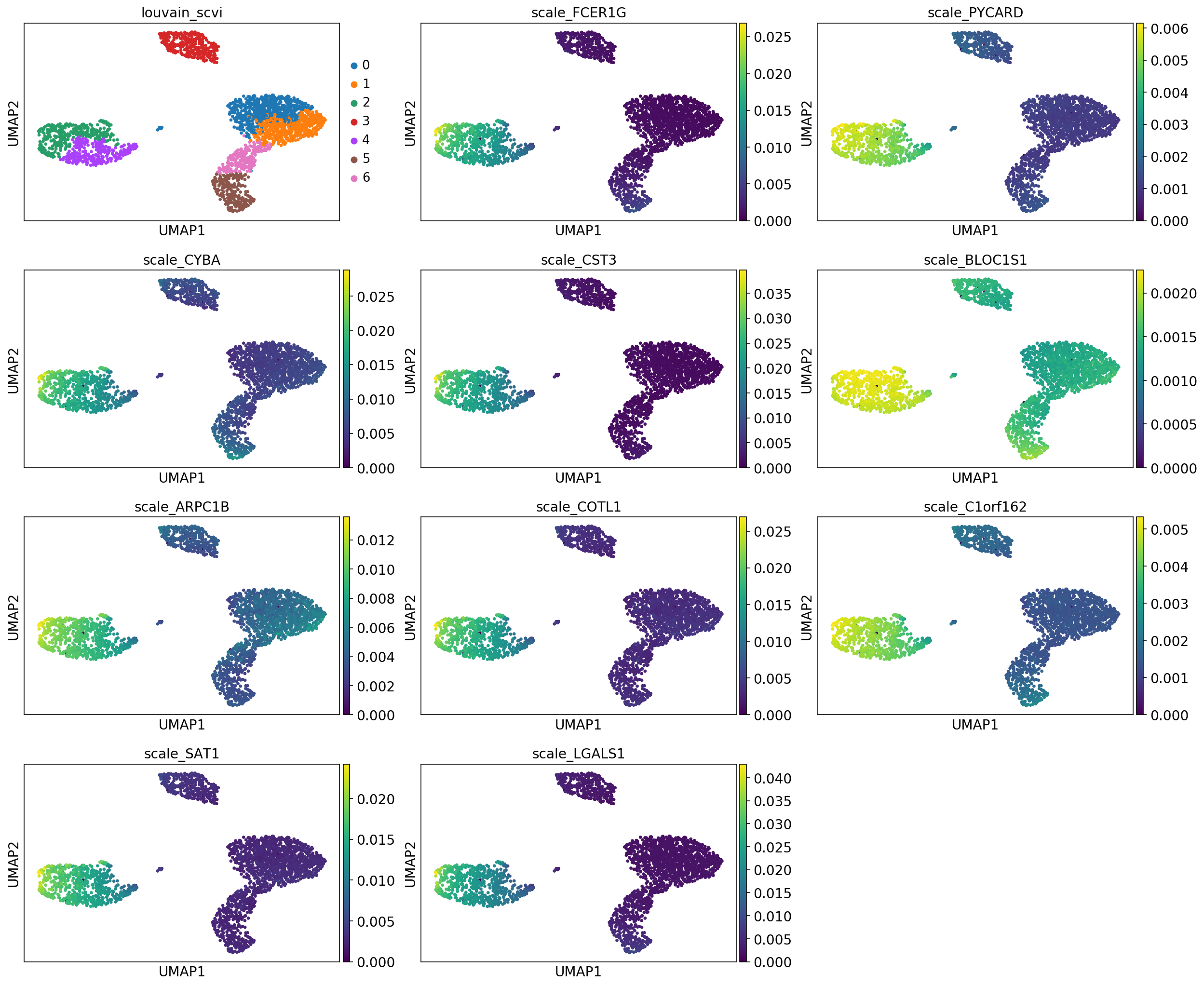
6.9. Plot px_scale for most expressed genes and less expressed genes by cluster¶
[30]:
cluster_id = 2
n_best_genes = 10
gene_names = differential_expression[
differential_expression["clusters"] == cluster_id
].index.tolist()[:n_best_genes]
gene_names
[30]:
['FCER1G',
'PYCARD',
'CYBA',
'CST3',
'BLOC1S1',
'ARPC1B',
'COTL1',
'C1orf162',
'SAT1',
'LGALS1']
[32]:
print("Top genes for cluster %d" % cluster_id)
sc.pl.umap(adata, color=["leiden_scvi"] + ["scale_{}".format(g) for g in gene_names], ncols=3, show=show_plot)
Top genes for cluster 2
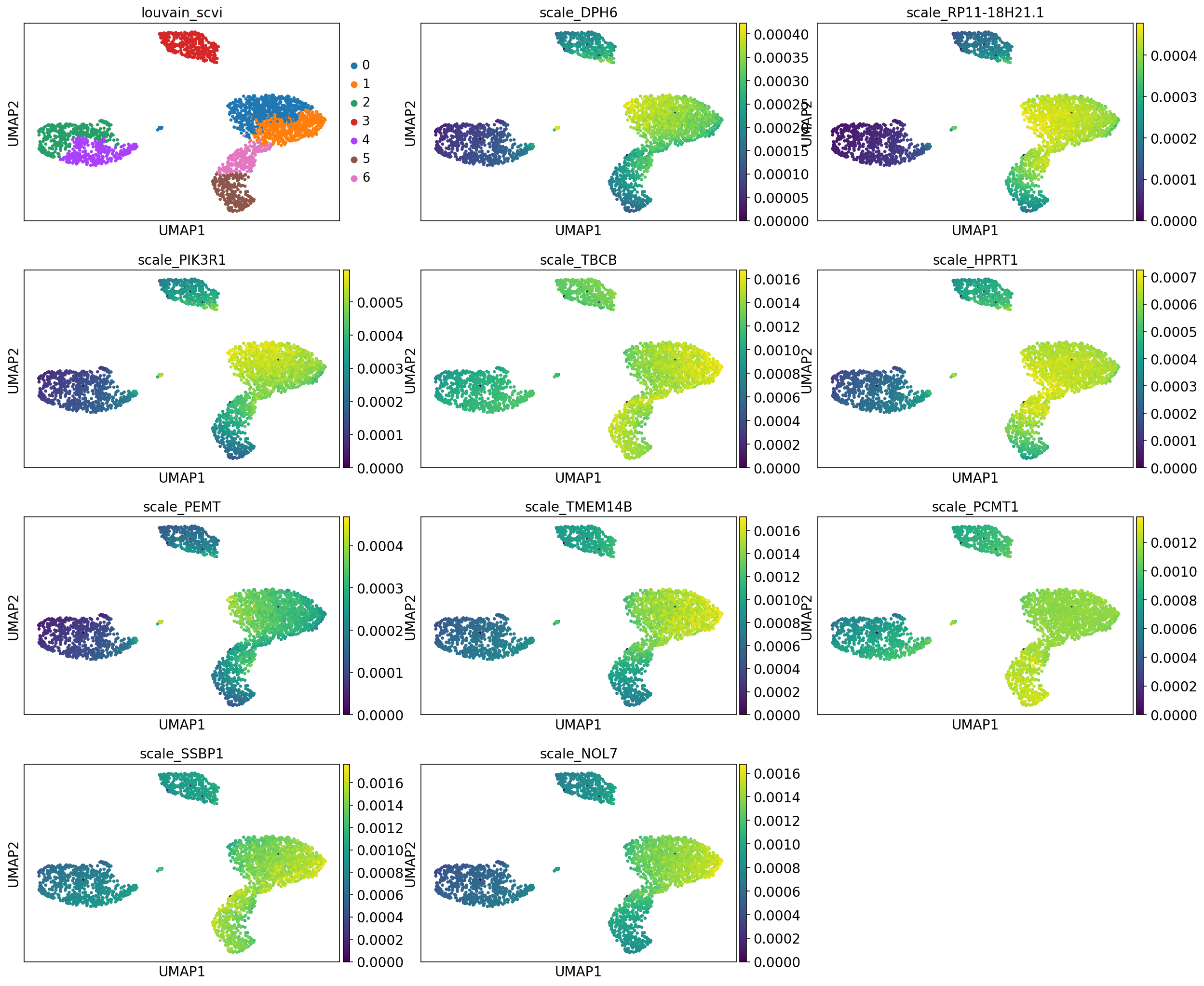
[33]:
cluster_id = 2
n_best_genes = 10
gene_names = differential_expression[
differential_expression["clusters"] == cluster_id
].index.tolist()[-n_best_genes:]
gene_names
[33]:
['DPH6',
'RP11-18H21.1',
'PIK3R1',
'TBCB',
'HPRT1',
'PEMT',
'TMEM14B',
'PCMT1',
'SSBP1',
'NOL7']
[35]:
print("Top down regulated genes for cluster %d" % cluster_id)
sc.pl.umap(adata, color=["leiden_scvi"] + ["scale_{}".format(g) for g in gene_names], ncols=3, show=show_plot)
Top down regulated genes for cluster 2
[40]:
def store_de_scores(
adata: sc.AnnData, differential_expression: pd.DataFrame, save_path: str = None
):
"""Creates, returns and writes a DataFrame with all the differential scores used in this notebook.
Args:
adata: scRNAseq dataset
differential_expression: Pandas Dataframe containing the bayes factor for all genes and clusters
save_path: file path for writing the resulting table
Returns:
pandas.DataFrame containing the scores of each differential expression test.
"""
# get shapes for array initialisation
n_genes_de = differential_expression[
differential_expression["clusters"] == 0
].shape[0]
all_genes = adata.shape[1]
# check that all genes have been used
if n_genes_de != all_genes:
raise ValueError(
"scvi differential expression has to have been run with n_genes=all_genes"
)
# get tests results from AnnData unstructured annotations
rec_scores = []
rec_names = []
test_types = ["ttest", "wilcox"]
for test_type in test_types:
res = adata.uns["rank_genes_groups_" + test_type]
rec_scores.append(res["scores"])
rec_names.append(res["names"])
# restrict scvi table to bayes factor
res = differential_expression[["bayes_factor", "clusters"]]
# for each cluster join then append all
dfs_cluster = []
groups = res.groupby("clusters")
for cluster, df in groups:
for rec_score, rec_name, test_type in zip(rec_scores, rec_names, test_types):
temp = pd.DataFrame(
rec_score[str(cluster)],
index=rec_name[str(cluster)],
columns=[test_type],
)
df = df.join(temp)
dfs_cluster.append(df)
res = pd.concat(dfs_cluster)
if save_path:
res.to_csv(save_path)
return res
[41]:
de_table = store_de_scores(adata, differential_expression, save_path=None)
de_table.head()
[41]:
| bayes1 | clusters | ttest | wilcox | |
|---|---|---|---|---|
| UCK1 | 2.456012 | 0 | 0.938648 | -0.266276 |
| SLC4A7 | 2.420804 | 0 | 1.226539 | -7.558761 |
| MDS2 | 2.412824 | 0 | 2.568651 | -7.237923 |
| NARG2 | 2.412824 | 0 | 1.782577 | -2.238907 |
| PDE6B | 2.407534 | 0 | -0.616006 | 4.964366 |
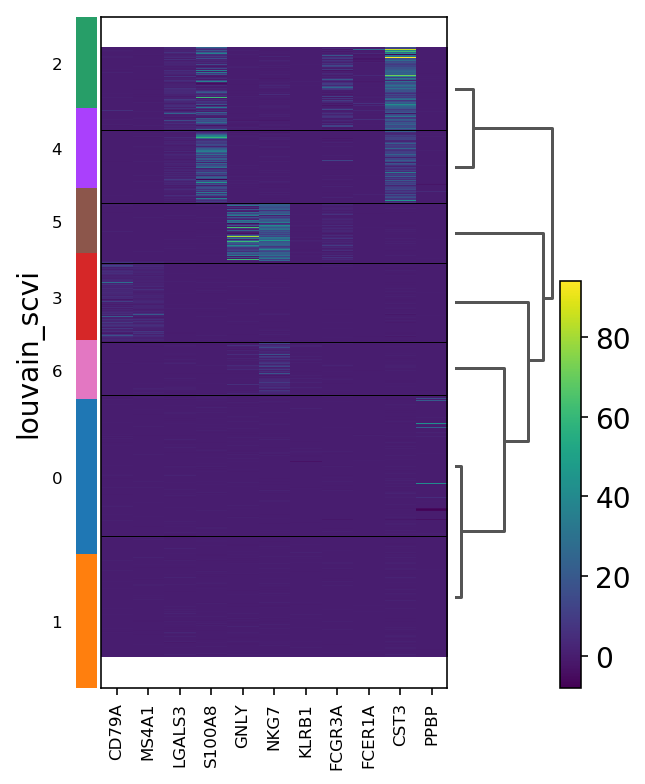
6.10. Running other ScanPy algorithms is easy, binding the index keys¶
[43]:
marker_genes = [
"CD79A",
"MS4A1",
"LGALS3",
"S100A8",
"GNLY",
"NKG7",
"KLRB1",
"FCGR3A",
"FCER1A",
"CST3",
"PPBP",
]
[44]:
sc.pl.heatmap(adata, marker_genes, groupby="leiden_scvi", dendrogram=True, show=show_plot)
[44]:
<matplotlib.gridspec.GridSpec at 0x7f821a5d20f0>
[ ]: