Atlas-level integration of lung data#
An important task of single-cell analysis is the integration of several samples, which we can perform with scVI. For integration, scVI treats the data as unlabelled. When our dataset is fully labelled (perhaps in independent studies, or independent analysis pipelines), we can obtain an integration that better preserves biology using scANVI, which incorporates cell type annotation information. Here we demonstrate this functionality with an integrated analysis of cells from the lung atlas integration task from the scIB manuscript. The same pipeline would generally be used to analyze any collection of scRNA-seq datasets.
Note
Running the following cell will install tutorial dependencies on Google Colab only. It will have no effect on environments other than Google Colab.
!pip install --quiet scvi-colab
from scvi_colab import install
install()
WARNING: Running pip as the 'root' user can result in broken permissions and conflicting behaviour with the system package manager. It is recommended to use a virtual environment instead: https://pip.pypa.io/warnings/venv
import os
import tempfile
import scanpy as sc
import scvi
import seaborn as sns
import torch
from rich import print
from scib_metrics.benchmark import Benchmarker
scvi.settings.seed = 0
print("Last run with scvi-tools version:", scvi.__version__)
Last run with scvi-tools version: 1.1.0
Note
You can modify save_dir below to change where the data files for this tutorial are saved.
sc.set_figure_params(figsize=(6, 6), frameon=False)
sns.set_theme()
torch.set_float32_matmul_precision("high")
save_dir = tempfile.TemporaryDirectory()
%config InlineBackend.print_figure_kwargs={"facecolor": "w"}
%config InlineBackend.figure_format="retina"
Note that this dataset has the counts already separated in a layer. Here, adata.X contains log transformed scran normalized expression.
adata_path = os.path.join(save_dir.name, "lung_atlas.h5ad")
adata = sc.read(
adata_path,
backup_url="https://figshare.com/ndownloader/files/24539942",
)
adata
AnnData object with n_obs × n_vars = 32472 × 15148
obs: 'dataset', 'location', 'nGene', 'nUMI', 'patientGroup', 'percent.mito', 'protocol', 'sanger_type', 'size_factors', 'sampling_method', 'batch', 'cell_type', 'donor'
layers: 'counts'
Dataset preprocessing#
This dataset was already processed as described in the scIB manuscript. Generally, models in scvi-tools expect data that has been filtered/aggregated in the same fashion as one would do with Scanpy/Seurat.
Another important thing to keep in mind is highly-variable gene selection. While scVI and scANVI both accomodate using all genes in terms of runtime, we usually recommend filtering genes for best integration performance. This will, among other things, remove batch-specific variation due to batch-specific gene expression.
We perform this gene selection using the Scanpy pipeline while keeping the full dimension normalized data in the adata.raw object. We obtain variable genes from each dataset and take their intersections.
adata.raw = adata # keep full dimension safe
sc.pp.highly_variable_genes(
adata,
flavor="seurat_v3",
n_top_genes=2000,
layer="counts",
batch_key="batch",
subset=True,
)
Important
We see a warning about the data not containing counts. This is due to some of the samples in this dataset containing SoupX-corrected counts. scvi-tools models will run for non-negative real-valued data, but we strongly suggest checking that these possibly non-count values are intended to represent pseudocounts, and not some other normalized data, in which the variance/covariance structure of the data has changed dramatically.
Integration with scVI#
As a first step, we assume that the data is completely unlabelled and we wish to find common axes of variation between the two datasets. There are many methods available in scanpy for this purpose (BBKNN, Scanorama, etc.). In this notebook we present scVI. To run scVI, we simply need to:
Register the AnnData object with the correct key to identify the sample and the layer key with the count data.
Create an SCVI model object.
scvi.model.SCVI.setup_anndata(adata, layer="counts", batch_key="batch")
We note that these parameters are non-default; however, they have been verified to generally work well in the integration task.
model = scvi.model.SCVI(adata, n_layers=2, n_latent=30, gene_likelihood="nb")
Now we train scVI. This should take a couple of minutes on a Colab session
model.train()
Epoch 246/246: 100%|██████████| 246/246 [04:21<00:00, 1.06s/it, v_num=1, train_loss_step=491, train_loss_epoch=552]
Once the training is done, we can evaluate the latent representation of each cell in the dataset and add it to the AnnData object
SCVI_LATENT_KEY = "X_scVI"
adata.obsm[SCVI_LATENT_KEY] = model.get_latent_representation()
Finally, we can cluster the dataset and visualize it the scVI latent space.
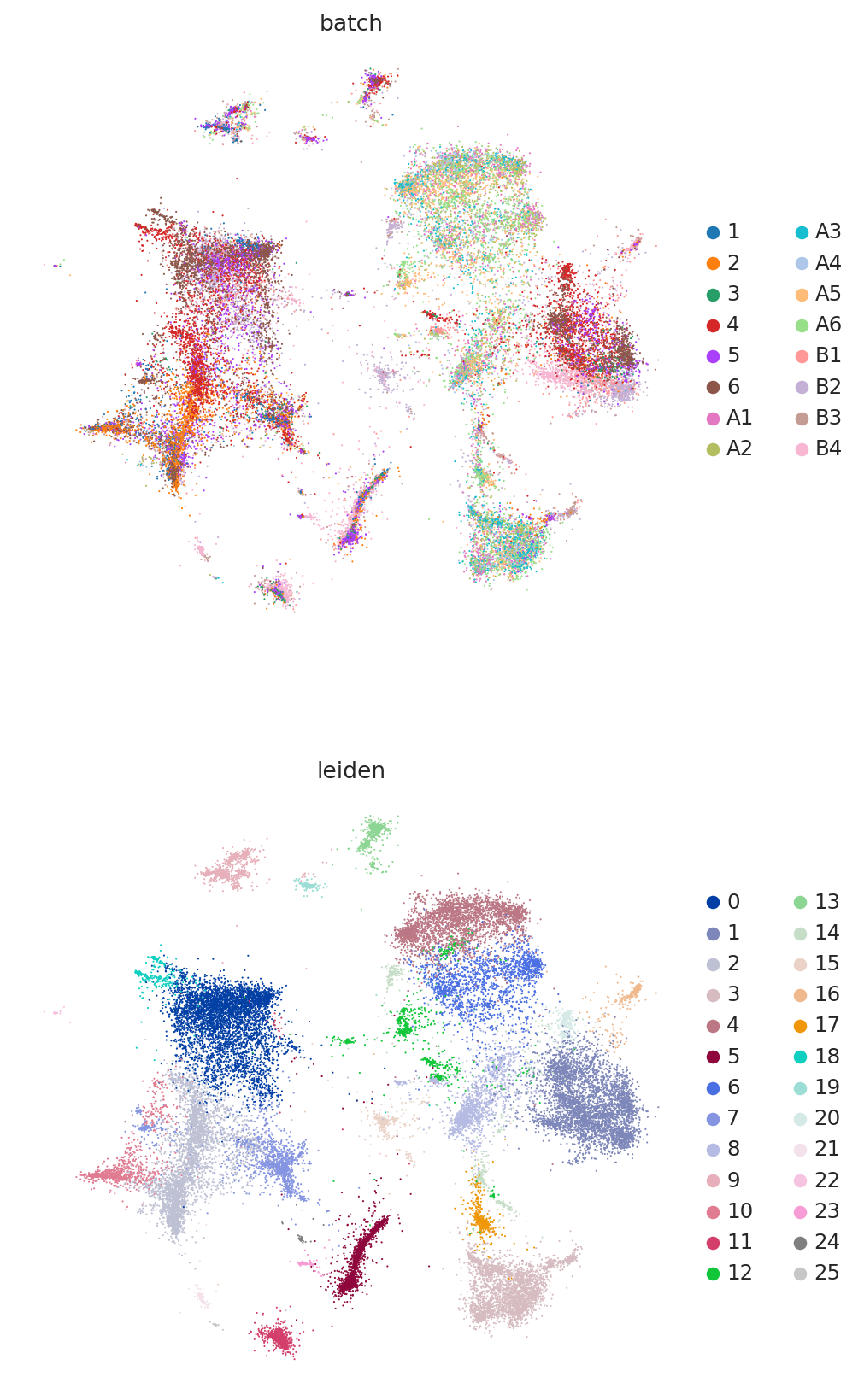
sc.pp.neighbors(adata, use_rep=SCVI_LATENT_KEY)
sc.tl.leiden(adata)
To visualize the scVI’s learned embeddings, we use the pymde package wrapperin scvi-tools. This is an alternative to UMAP that is GPU-accelerated.
SCVI_MDE_KEY = "X_scVI_MDE"
adata.obsm[SCVI_MDE_KEY] = scvi.model.utils.mde(adata.obsm[SCVI_LATENT_KEY])
INFO Using cuda:0 for `pymde.preserve_neighbors`.
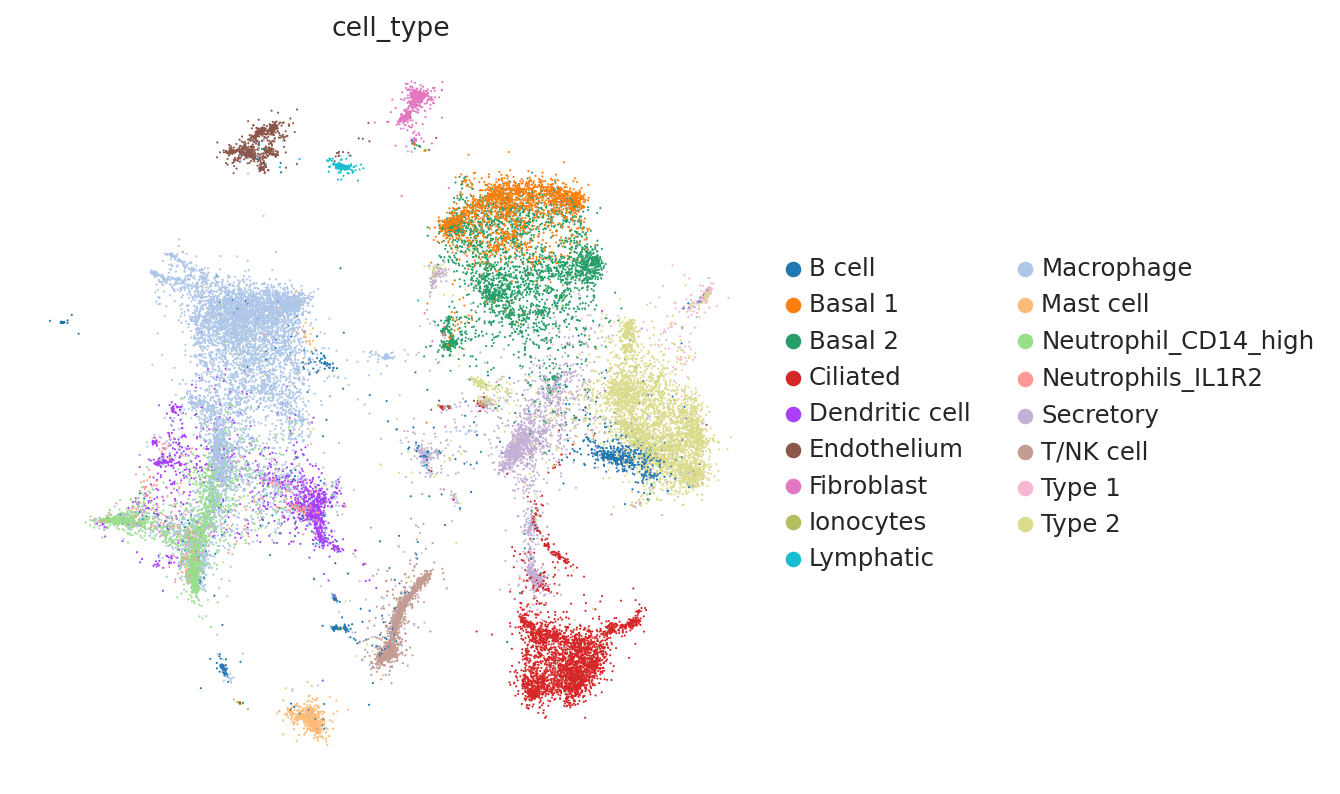
Because this data has been used for benchmarking, we have access here to curated annotations. We can use those to assess whether the integration worked reasonably well.
At a quick glance, it looks like the integration worked well. Indeed, the two datasets are relatively mixed in latent space and the cell types cluster together. We see that this dataset is quite complex, where only some batches contain certain cell types.
Below we quantify the performance.
Integration with scANVI#
Previously, we used scVI as we assumed we did not have any cell type annotations available to guide us. Consequently, after the previous analysis, one would have to annotate clusters using differential expression, or by other means.
Now, we assume that all of our data is annotated. This can lead to a more accurate integration result when using scANVI, i.e., our latent data manifold is better suited to downstream tasks like visualization, trajectory inference, or nearest-neighbor-based tasks. scANVI requires:
the sample identifier for each cell (as in scVI)
the cell type/state for each cell
scANVI can also be used for label transfer and we recommend checking out the other scANVI tutorials to see explore this functionality.
Since we’ve already trained an scVI model on our data, we will use it to initialize scANVI. When initializing scANVI, we provide it the labels_key. As scANVI can also be used for datasets with partially-observed annotations, we need to give it the name of the category that corresponds to unlabeled cells. As we have no unlabeled cells, we can give it any random name that is not the name of an exisiting cell type.
Important
scANVI should be initialized from a scVI model pre-trained on the same exact data.
scanvi_model = scvi.model.SCANVI.from_scvi_model(
model,
adata=adata,
labels_key="cell_type",
unlabeled_category="Unknown",
)
scanvi_model.train(max_epochs=20, n_samples_per_label=100)
INFO Training for 20 epochs.
Epoch 20/20: 100%|██████████| 20/20 [01:00<00:00, 3.03s/it, v_num=1, train_loss_step=506, train_loss_epoch=534]
Now we can retrieve the latent space
SCANVI_LATENT_KEY = "X_scANVI"
adata.obsm[SCANVI_LATENT_KEY] = scanvi_model.get_latent_representation(adata)
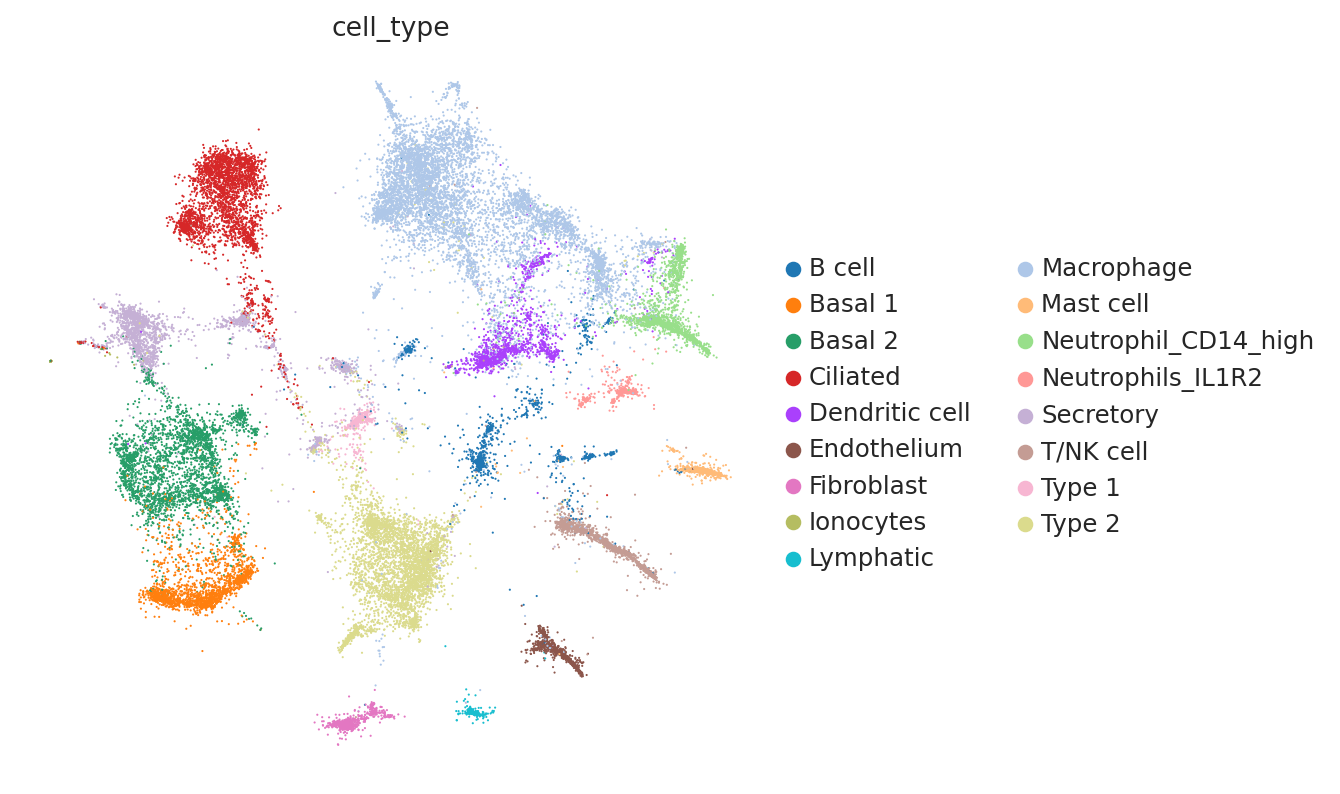
Again, we may visualize the latent space as well as the inferred labels
SCANVI_MDE_KEY = "X_scANVI_MDE"
adata.obsm[SCANVI_MDE_KEY] = scvi.model.utils.mde(adata.obsm[SCANVI_LATENT_KEY])
INFO Using cuda:0 for `pymde.preserve_neighbors`.
Compute integration metrics#
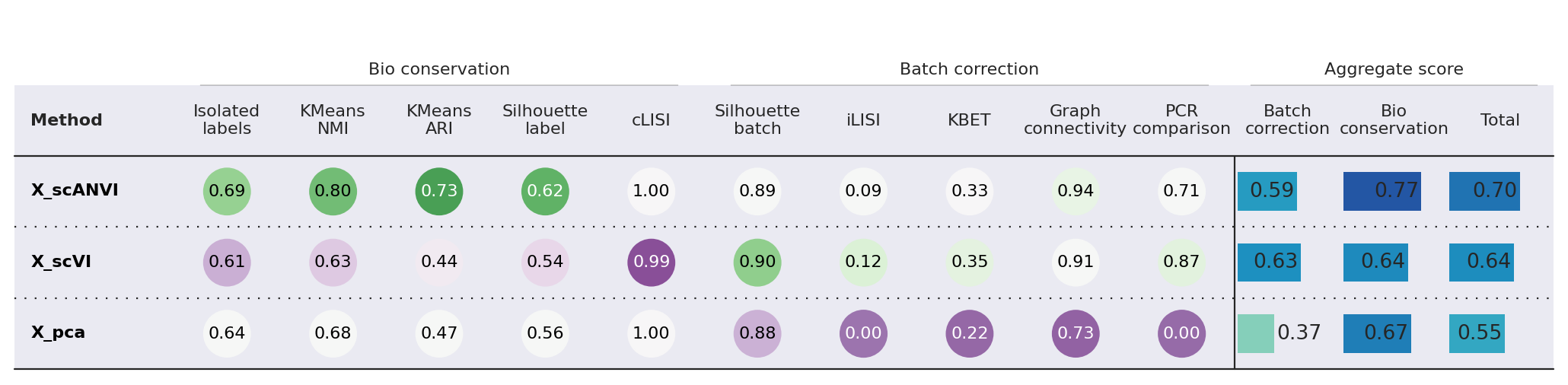
Here we use the scib-metrics package, which contains scalable implementations of the metrics used in the scIB benchmarking suite. We can use these metrics to assess the quality of the integration.
We can see that the additional training with label information and scANVI improved the metrics that capture bio conservation (cLISI, Silhouette labels) without sacrificing too much batch correction power (iLISI, Silhouette batch)
bm = Benchmarker(
adata,
batch_key="batch",
label_key="cell_type",
embedding_obsm_keys=["X_pca", SCVI_LATENT_KEY, SCANVI_LATENT_KEY],
n_jobs=-1,
)
bm.benchmark()
df = bm.get_results(min_max_scale=False)
print(df)
Isolated labels KMeans NMI KMeans ARI \ Embedding X_pca 0.64364 0.681808 0.470352 X_scVI 0.607581 0.625488 0.44097 X_scANVI 0.687845 0.804745 0.732296 Metric Type Bio conservation Bio conservation Bio conservation Silhouette label cLISI Silhouette batch \ Embedding X_pca 0.557418 1.0 0.878176 X_scVI 0.536606 0.993054 0.895216 X_scANVI 0.624912 1.0 0.885531 Metric Type Bio conservation Bio conservation Batch correction iLISI KBET Graph connectivity \ Embedding X_pca 0.003445 0.223596 0.728504 X_scVI 0.117146 0.34879 0.910749 X_scANVI 0.089942 0.327317 0.939452 Metric Type Batch correction Batch correction Batch correction PCR comparison Batch correction Bio conservation \ Embedding X_pca 0.0 0.366744 0.670644 X_scVI 0.868805 0.628141 0.64074 X_scANVI 0.711203 0.590689 0.769959 Metric Type Batch correction Aggregate score Aggregate score Total Embedding X_pca 0.549084 X_scVI 0.6357 X_scANVI 0.698251 Metric Type Aggregate score